Fig. 1
Receptor autoradiography showing 177Lu-DOTA-BASS antagonist versus 177Lu-DOTATATE agonist binding in renal cancer (upper row), breast cancer (middle row), and non-Hodgkin lymphoma (lower row). a Total binding with the respective radioligands; b nonspecific binding. Bars = 1 mm
3 Bombesin Receptors
Peptide receptor targeting of tumors is normally based on overexpression of receptors in tumor cells. In the GRP receptor field, this is well documented for prostate and breast cancers (Gugger and Reubi 1999; Markwalder and Reubi 1999). However, we have observed recently that a variety of human tumors can demonstrate massive overexpression of GRP receptors in tumoral and peritumoral vessels, while they rarely express GRP receptors in the tumor cell. This is most impressive in ovarian cancers and urinary tract cancers (Fleischmann et al. 2007, 2009).
We showed for instance that in ovarian carcinomas the GRP receptor protein was expressed in tumoral blood vessels in 84% of cases, but in tumor cells in only 32% of cases (Fleischmann et al. 2007). In contrast, normal ovarian vessels do not express measurable amounts of GRP receptors. GRP-receptor-expressing blood vessels are clearly limited to the tumors and the tumoral interface. These vessels are mainly of small size (30–120 μm) and possess a muscular coat indicating mature vessels; the GRP receptors are usually expressed in high densities, and homogeneously and transmurally distributed in the muscular wall of the vessels (Fleischmann et al. 2007). As muscle cells have the ability to contract, these vascular receptors might play a role in hemodynamics, influencing tumor progression by alteration of the blood flow in the tumoral vasculature. Indeed, bombesin and GRP have been shown to influence the vascular tone under physiological conditions (Bjenning et al. 1991; Clive et al. 2001; Luu et al. 1993); treatment with GRP-receptor antagonists can alter the microvascular perfusion of a tumor in a xenograft model (Heuser et al. 2005). Alternatively, overexpression of GRP receptors in this site might be implicated in blood vessel maturation, defined as the process of recruitment of cells (pericytes, smooth muscle cells) to form the vessel wall, which is an important step in neoangiogenesis. This step involves distinct mechanisms such as cell proliferation, migration, and morphogenesis, all known to be mediated by GRP and the corresponding receptor (Bunnett 1994; Jensen et al. 2001; Kim et al. 1997; Yule and White 1999). The results of recent functional studies propose GRP to be a new angiogenic molecule and link GRP and its receptor to tumor neoangiogenesis; for instance, GRP upregulates the proangiogenic vascular endothelial growth factor (VEGF) A in vitro in prostate and endometrial cancer cell lines (Levine et al. 2003a, b).
GRP-receptor-positive blood vessels are present in all ovarian tumor categories, with a tendency for higher incidence of GRP-receptor-positive vessels in malignant tumors compared with benign or low-grade malignant tumors (Fleischmann et al. 2007). This finding might reflect the different conditions of vascular development in various tumors with variable growth velocities. Most interestingly, these GRP-receptor-positive vessels are detected in particularly high density in tumors of ovarian origin that are usually fatal, namely metastases of primary ovarian carcinomas. This phenomenon points to GRP receptor overexpression in tumor vasculature as a mechanism independent of the primary site of tumor genesis. Interestingly, these vessels are present in very high numbers and with high GRP receptor densities in these metastases, potentially reflecting tumor clones with uppermost angiogenic properties (Fleischmann et al. 2007).
In a follow-up study, selected other human cancers also showed high prevalence of GRP receptors in their tumoral vessels. This is particularly true for invasive urothelial carcinomas, but also for squamous lung carcinomas, colorectal carcinomas, renal cell carcinomas, or lung adenocarcinomas (Fleischmann et al. 2009).
Recently, we further characterized these GRP receptors by comparing the vascular GRP receptor expression and localization in a selection of human cancers with that of an established biological marker of neoangiogenesis, the VEGF receptor (Reubi et al. 2011). In vitro quantitative receptor autoradiography was performed in parallel for GRP and VEGF receptors in 32 human tumors of various origins, using 125I-Tyr-bombesin and 125I-VEGF165 as radioligands, respectively. While all tumors expressed GRP and VEGF receptors in their vascular system, VEGF receptors were expressed in the endothelium in the majority of the vessels and GRP receptors were expressed in a subpopulation of vessels, preferably in their muscular coat (Reubi et al. 2011). The vessels expressing GRP receptors were all VEGF receptor positive, whereas the VEGF-receptor-expressing vessels were not all GRP receptor positive. GRP-receptor-expressing vessels were found immunohistochemically to coexpress VEGF receptor subtype 2. Remarkably, the density of vascular GRP receptors was much higher than that of VEGF receptors. A representative example is shown in Fig. 2. Concomitant expression of GRP and VEGF receptors appears to be a frequent phenomenon in many human cancers. The GRP receptors, localized and expressed in extremely high density in a subgroup of vessels, may function as target for antiangiogenic tumor therapy or angiodestructive targeted radiotherapy with radiolabeled bombesin analogs given alone or preferably together with VEGF-receptor-targeted therapy.
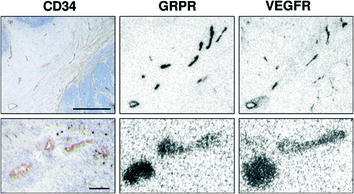

Fig. 2
Tumoral and peritumoral vessels express both GRP receptors and VEGF receptors in autoradiographic experiments. CD34 immunohistochemistry identifies tumoral and peritumoral vessels. Bars = 1 mm (upper row) or 0.1 mm (lower row)
GRP receptor overexpression is the molecular basis for in vivo targeting with radiolabeled bombesin analogs. Clinical proof of principle has been obtained in vivo for targeting GRP-receptor-overexpressing breast and prostate cancers, with a 99Tc-labeled bombesin analog. Further, second-generation bombesin radioligands have been developed, such as 177Lu-DOTA-CH2CO-G-4-aminobenzoyl-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2 (177Lu-AMBA) or 90Y-DOTA-PEG4-bombesin(7-14) (90Y-DOTA-PESIN), which are bombesin agonists characterized by excellent receptor-mediated internalization into the cell and, therefore, high tumor accumulation (Reubi and Maecke 2008). Recently, however, it was found that radiolabeled GRP receptor antagonists, although they do not internalize easily, are even better tumor markers than agonists (Cescato et al. 2008; Mansi et al. 2009, 2011). Targeted tumor radiotherapy with potent bombesin radiolabeled antagonists may not only include destruction of tumor cells, but also of tumoral and peritumoral vessels, or both, and as such may become a powerful alternative to current oncologic therapy.
Interestingly, despite the strong in vitro evidence for GRP receptor expression in tumoral vessels and the availability of excellent bombesin radioligands, there has been no in vivo study in patients evaluating whether metastatic ovarian cancers or urinary tract cancers can be targeted through their overexpressed vascular GRP receptors. Such a study, even though logistically difficult, is greatly awaited.
4 Incretin Receptors
Incretins such as GLP-1 or glucose-dependent insulinotropic polypeptide (GIP) are important glucose-dependent insulin secretagogues released primarily from the gastrointestinal tract in response to nutrient intake (Holst 2007; Holst et al. 2009; Irwin and Flatt 2009). In addition to their regulation of glucose-dependent insulin secretion, those peptides have other common actions on β-cells, including stimulation of cell proliferation and reduction of β-cell apoptosis (Ehses et al. 2003; Holst et al. 2009; Kim et al. 2008). These effects are mediated by specific GLP-1 and GIP receptors in pancreatic islets. Interestingly, recent data indicate that incretin receptors can also be overexpressed in selected human cancers; for instance, extremely high GLP-1 receptor expression has been reported in insulinomas (Reubi and Waser 2003), leading to the successful development of GLP-1 receptor targeting in these tumors (Christ et al. 2009; Wild et al. 2008, 2011a). Moreover, we recently observed that, unexpectedly, medullary thyroid carcinomas, a tumor originating from an organ unrelated to incretin physiological action, expresses incretin receptors to various extents; GLP-1 receptors are expressed in 27% of medullary thyroid carcinomas, while GIP receptors are expressed in as many as 89% of these carcinomas (Korner et al. 2007; Waser et al. 2011).
The unexpectedly high GIP receptor expression in human medullary thyroid carcinomas might be the molecular basis for GIP receptor targeting in vivo in those patients. At the moment, GIP-derived radioligands are not available for preclinical studies; however, the development of such compounds may be greatly facilitated by our findings of high GIP receptor expression in the TT cell line (Waser et al. 2011).
We may expect that, in the future, incretin receptors, in particular GIP receptor targeting of medullary thyroid carcinomas, may be combined with cholecystokinin 2 receptor targeting that has previously been reported to be successful in imaging medullary thyroid carcinomas in patients, due to the massive overexpression of cholecystokinin 2 receptors in these tumors (Reubi 2007). Figure 3 compares the incidence and density of the three receptors in imaging medullary thyroid carcinomas.
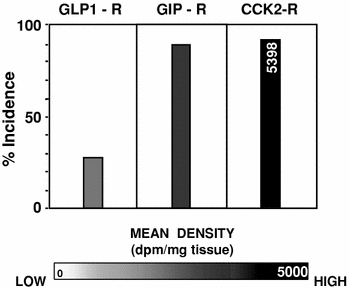

Fig. 3




Incidence and density of GLP-1 receptors, GIP receptors, and cholecystokinin 2 receptors in medullary thyroid carcinomas. Mean density from 0 to 5,000 dpm/mg is represented by the darkness of the bars. Values for mean density exceeding 5,000 dpm/mg tissue are reported in the bars
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree