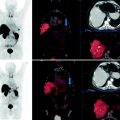
Fig. 1
Schematic illustrating traditional approach compared with receptor targeting
A disadvantage of the conventional bifunctional chelating system is that only one radioactive molecule per targeting moiety at most is delivered. This is highly dependent on the specific activity of the radioisotope and on any metal contaminants, as many chelators are not selective but will bind to a number of common metals. A low specific activity and/or high presence of unwanted metal impurities can result in low labeling yields for the desired radioisotope and saturation of the receptor sites if they are present in low quantities. This has been recently demonstrated by Asti et al. (2010), who performed a systematic study evaluating the effect of specific activity and incorporation of a variety of metals on a typical DOTATOC clinical formulation.
2 Nanoparticles
Radioisotopes of gold and other coinage metals have nuclear properties that make them ideal for use in imaging and therapeutic applications. However, their biologically active redox chemistry and lack of stable chelating agents have to-date limited their utilization in vivo. They have experienced some use in the area of brachytherapy with the bare metal being encapsulated, but otherwise have been used sparingly, the major concern being the inherent toxicities observed for the free metals in vivo. In order to take advantage of their nuclear properties, we have been seeking alternative means of delivering radioisotopes more efficaciously. A novel approach is the development of metal nanoparticles that can be derivatized on their surface, allowing for optimization of biodistribution in vivo.
Once a metal nanoparticle is fabricated, its in vivo properties can be evaluated and altered by varying such properties as its size, charge, hydrodynamic size, organic surface layer, and shape. These can be optimized to enable more favorable uptake and clearance in vivo. Furthermore, due to their high surface area, they can be conjugated with a plethora of agents that can be used for selective targeting such as peptides and small molecules or agents either to enhance retention in the blood stream or to enhance clearance. These properties can be optimized through in vivo studies to develop lead compounds for imaging and therapy. A major advantage of nanoparticles over the commonly used bifunctional chelator approach is that the nanoparticle platform allows for much higher payload delivery, as more than one radioisotope can be incorporated into the nanoparticle and can result in a much higher dose delivery even with a low specific activity radionuclide.
2.1 Gold Radioisotope Properties
Radioisotopes of gold (Au) as shown in Table 1 have attractive nuclear properties that make them ideal for imaging and therapy. Two radioisotopes of gold stand out; one of which is 198Au, which has a moderate-energy beta particle (0.96 keV β− max), making it a good candidate for therapy, and more than 90% emission of a 412-keV gamma emission that allows for in vivo tracking and dosimetry calculation. These properties led to its initial use as a brachytherapy agent for prostate cancer. A second radioisotope, 199Au, has a low-energy beta maximum (0.46 MeV) and emits a 158-keV gamma with 36% yield that is easily detected by SPECT cameras. Additionally, 199Au can be produced carrier free by neutron irradiation of 198Pt to produce 199Pt that decays in 3.2 min to 199Au.
Table 1
Properties of gold radioisotopes
Radionuclide | Half-life | Max β-energy (MeV) | Max γ-energy (keV) |
|---|---|---|---|
Au-198 | 2.7 days | 0.96 (99%), E av = 0.315 | 411 |
Au-199 | 3.2 days | 0.46, E av = 0.14 | 158 |
Gold nanoparticles (AuNPs) have been around for quite some time, but their harsh production methods with toxic chemicals have prevented them from being attached easily to biomolecules for evaluation in vivo. A majority of metal nanoparticles are made with toxic chemicals that are not conducive for in vivo applications. Early on we evaluated safe, green methods to produce gold nanoparticles in aqueous solutions that would be amenable to use in biomolecular applications.
2.2 Gum Arabic Gold Nanoparticles
Our first synthesis consisted of using THPAL, a trimeric phosphino alanine, to reduce gold salts in water containing green organics, which would coat the surface of the gold nanoparticles and form 12–15 nm-sized gold nanoparticles with hydrodynamic diameter of 60–85 nm (Kannan et al. 2006). These methods have been shown to be robust and nontoxic in the nonradioactive form. The methods have been altered to allow for formation of both 198Au and 199Au nanoparticles and also have been shown amenable to formation with other coin metals such as palladium and silver. We have developed a library of such conjugates, three of which have been evaluated in induced tumor models and two are being translated into large animals for imaging and treatment of prostate cancer.
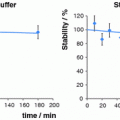
Gum arabic-coated gold nanoparticles (GA-198AuNPs) were the first and most studied to date (Chanda et al. 2010). The reaction consists of heating water containing gum arabic, adding gold either as the NaAuCl4 salt or the H198AuCl4 acid along with the THPAL, heating this solution briefly, which converts from a pale-yellow solution to a red-burgundy one. Quality control has shown that this method results in 99% conversion of the radioactive gold to the nanoparticle form, which then can be brought to neutral pH and remains stable in the radioactive form for over 2 weeks. This formulation was initially evaluated for therapeutic efficacy in SCID mice bearing induced human prostate tumors. Different modes of injection were tried, with intratumoral injection resulting in the highest uptake and retention. These studies prompted us to evaluate their efficacy in tumor stabilization in the same tumor models. Tumors received a 70 Gy dose by administering 405 μCi. An overall 82% reduction in tumor volume was noted between the mice receiving GA 198AuNP compared with controls receiving saline injections. Biodistributions at the end showed a reduction of gold nanoparticles in the tumor from 70% at 24 h to 19.9 ± 4.2% ID at 30 days post injection. Clearance was predominantly through the urinary track, and minimum uptake was observed in other organs as can be seen in Table 2. Based on these results, these nanoparticles are being evaluated for their therapeutic efficacy in canines with spontaneous prostate cancer. Prostate cancer in dogs has been shown to mimic that observed in humans on the functional level as well as in metastatic rate and occurrence. Studies to date have shown that dogs treated with the nanoconstructs tolerate the treatment well and that the disease is stabilized.
Table 2
Comparison of uptake in selected tissues for GA-198AuNP and EGCg-198AuNP
Tissue | GA-198AuNP | EGCg-198AuNP |
|---|---|---|
Liver | 0.91 ± 0.26% ID | 2.5 ± 1.7% ID |
Kidney | 0.13 ± 0.01% ID | Background |
Small intestines | 0.09 ± 0.00% ID | Background |
Tumor | 19.9 ± 4.2% ID | 37.4 ± 1.8% ID |
Carcass | 18.5 ± 4.6% ID | 17.8 ± 6.1% ID |
2.3 Epigallocatechin-Gallate Gold Nanoparticles
Another formulation was a receptor targeted approach which was developed using the Food and Drug Administration (FDA)-approved photochemical epigallocatechin-gallate (EGCg) (Shukla et al. 2011). EGCg is a major phytochemical component of green tea and has been used extensively as a food supplement because of its strong antioxidant properties. This method proved much simpler for two reasons: the nanoparticles could be formed at room temperature, and EGCg not only achieved reduction of the gold but also served as a stabilizing agent, resulting in an EGCg-conjugated gold nanoparticle (EGCg-198AuNPs) formulation in a few minutes at room temperature in water. Additionally EGCg targets the LAMININ receptor (Lam 67R) which is overexpressed on human prostate cancer cells (Tachibana et al. 2004). Lam 67R is a 67-kDa cell surface protein, first isolated as a non-integrin high-affinity laminin binding protein from murine cancer cells in 1983 by two independent research groups (Rao et al. 1983; Malinoff and Wicha 1983). EGCg has been known to bind to Lam 67R with excellent specificity and selectivity (Tachibana et al. 2004). The in vivo biodistribution of EGCg-198AuNPs was evaluated in SCID mice bearing human prostate cancer tumors and was shown to have an initial higher retention and to clear with time. Based on these results, they were evaluated in therapy trials with SCID mice bearing human prostate cancer. Due to the higher initial uptake, only a third of the dose activity (136 versus 405 μCi for the gold nanoparticles formed with gum arabic) needed to be injected to give a 70 Gy dose. The EGCg-198AuNPs were shown to be more stable over time, remaining in solution far longer than their gum arabic analogs, and results showed similar reduction of tumor volumes (80% compared with 82%) with higher clearance from normal tissues except for the liver and the tumor, as can be seen in Table 2. From the data it is clear that selective targeting of the EGCg to the Lam 67R receptor results in an overall higher uptake and retention in the prostate tumor cells than GA-198AuNP-treated ones. Due to the similar results with a smaller dose, these nanoparticles will also be evaluated in canine patients with spontaneous prostate cancer.
2.4 Bombesin Gold Nanoparticles
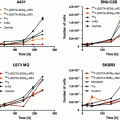
In addition to glycoprotein- and phytochemical-conjugated gold nanoparticles, we have also evaluated selective targeting by conjugating the 14-amino-acid peptide bombesin to the gold nanoparticle surface (McLaughlin et al. 2011a; Chanda et al. 2010). Bombesin (BBN) targets the gastrin-releasing peptide (GRP) receptors that are upregulated in a variety of cancers, predominantly breast, prostate, pancreatic, and lung cancers (Smith et al. 2003). The hypothesis was that attaching BBN to gold nanoparticles would result in selective uptake in prostate cancer, particularly metastases. Different formulations of gold nanoparticles conjugated to BBN (AuNP-BBN) with varying ratios of BBN to gold nanoparticles of 1:1, 2:1, and 3:1 were prepared and evaluated for their target specificity. AuNP-BBN conjugates evaluated through receptor binding assays demonstrated that the 1:3 AuNP-BBN conjugate showed the highest affinity and uptake (Chanda et al. 2010). This conjugate (BBN198AuNP) was evaluated in SCID mice bearing human prostate PC-3 cells and in a spontaneous model of prostate cancer, the TRAMP mouse. Although the initial studies in PC-3 hinted at selective uptake, more thorough evaluation in which the uptake was compared with 64Cu-NOTA-8-Aoc-BBN(7-14)NH2 showed only nonselective uptake. Starch gold nanoparticles (S198AuNP) were used as starting material to conjugate the BBN peptide. S198Au-NPs were conjugated to BBN by use of BBN 7-14 conjugated to thioctic acid. 64Cu-NOTA-8-Aoc-BBN(7-14)NH2 was prepared as previously described (Prasanphanich et al. 2007). We used SCID mice bearing a flank model of human prostate cancer derived from a subcutaneous implant of PC-3 cells for pharmacokinetic studies. 64Cu-NOTA-8-Aoc-BBN(7-14)NH2 injected intravenously (i.v.) exhibited high uptake in the pancreas and tumor, similar to what has been previously reported; this uptake was reduced by pretreatment with cold BBN. The intraperitoneal (i.p.) route of administration of the agent showed similar accumulation in tissues except the tumor, which was lower and was not blocked by the cold BBN. However, other receptor-rich tissues such as the pancreas showed reduced uptake (p ≤ 0.05). GA198Au-NP when injected i.p. showed no tumor uptake and no differences when injected with the blocking dose of BBN. Starch 198Au-NP showed no tumor uptake and no reduction in receptor-rich tissues when injected with cold BBN. The BBN-conjugated starch 198Au-NP showed similar results with no uptake observed in the tumor and no significant difference in uptake in the receptor-rich pancreas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree