Fig. 1
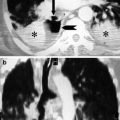
Thoracic aortic aneurysm in a 59-year-old man. Contrast-enhanced coronal MIP image (a) and sagittal MPR image (b) show a true aneurysm, with fusiform dilatation of the descending aorta
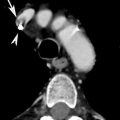
Fig. 2
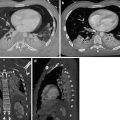
PA and lateral X-rays (a, b) show widened mediastinum, opacification of the aortopulmonary window, and rightward deviation of the trachea. Axial (c), coronal (d), and sagittal (e) MPR CT images show saccular dilatation of the proximal descending aorta
Fig. 3
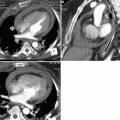
Marfan syndrome and annuloaortic ectasia in a 42-year-old woman. (a) T1-weighted and (b) gradient echo sagittal oblique MRI show a pear-shaped aorta that tapers to a normal aortic arch, a characteristic finding in Marfan syndrome
1.3.2 Pathophysiology
The pathogenesis of aneurysm is extremely complex and not completely understood; most likely, aneurysms result from the interaction of multiple factors, systemic and local hemodynamic features (Michel et al. 2011). The elastic properties of the aorta are important for its normal function. The elasticity of the wall allows the aorta to accept the pulsatile output of the left ventricle in systole and to modulate continued forward flow during diastole. With aging, the medial elastic fibers become thinned and fragmented, with the increase in collagen and ground substance. Elasticity and compliance of the aortic wall are progressively lost, with the increase in pulse pressure and with progressive dilatation of the aorta (Goldstein et al. 2015). Many authors suggest that a group of enzymes called matrix metalloproteinases (MMPs) plays a significant role in the destruction of extracellular matrix in the aortic wall. MMPs lead to the degradation of these structural proteins with elastic fiber fragmentation and loss and degeneration of the tunica media with loss of elasticity and weakening of the aortic wall and consequent dilation (Sakalihasan et al. 2005). Hemodynamic factors probably play a fundamental role in the formation of aortic aneurysms. The human aorta is a relatively low-resistance circuit for circulating blood while the lower extremities have higher arterial resistance (Hoshina et al. 2003). Repeated hydrostatic trauma may injure a diseased aortic wall and contribute to aneurysmal development. In case of concomitant systemic hypertension, it can accelerate the expansion of known aneurysms and may contribute to their initial formation (Piccinelli et al. 2013). Wall rupture follows the basic principles of material failure, i.e., an aneurysm breaks open when mural stress or deformation meets an appropriate failure criterion. The law of Laplace states that wall tension is proportional to the pressure and to the radius of the arterial conduit (T = P × R). As diameter increases, wall tension increases, which contributes to increasing diameter and risk of rupture. Also increased pressure and aneurysm size aggravate wall tension and therefore increase the risk of rupture. The use of the law of Laplace to predict AAA rupture potential is erroneous, because the AAA wall geometry is not a simple cylinder or sphere with a single radius of curvature, and wall stress alone is not sufficient to predict AAA rupture. So AAA diameter cannot be considered as the only determinant factor of either wall strength or wall stress (Piccinelli et al. 2013). Various indices have been proposed to create a more reliable and patient-specific criteria for clinical practice. Despite many progresses, the majority of the proposed criteria for risk stratification and decision making still rely on empirical measurements rather than on the analysis of biomechanical properties (Creasy et al. 1997); among these are maximum AAA diameter (Greenhalgh 2004), currently the prevalent index for the evaluation of risk of rupture; AAA expansion rate (Hirose and Takamiya 1998); wall stiffness (Fillinger et al. 2003); intraluminal thrombus thickness (Speelman et al. 2010); and AAA wall peak stress (Raghavan et al. 2000; McGloughlin and Doyle 2010). The presence of anisotropic displacement of the AAA lumen boundary and their link to hemodynamic forces have been assessed, highlighting a new possible role for hemodynamics in the study of AAA progression (Piccinelli et al. 2013).
1.4 Etiology
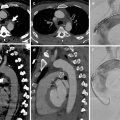
Atherosclerosis is the cause of approximately 70 % of all TAAs (Lesko et al. 1997) and contributes to the formation of aortic aneurysms secondary to the degeneration of the components of the aortic wall. Atherosclerotic aneurysms are the most common type; usually they are seen in the elderly and typically characterized by intimal calcification and fibrous plaques along the aorta. The majority of them are fusiform, up to 20 % may be saccular (Posniak et al. 1990), and they occur in the descending thoracic aorta and rarely involve only the ascending aorta (Lesko et al. 1997) (Fig. 4a, b).
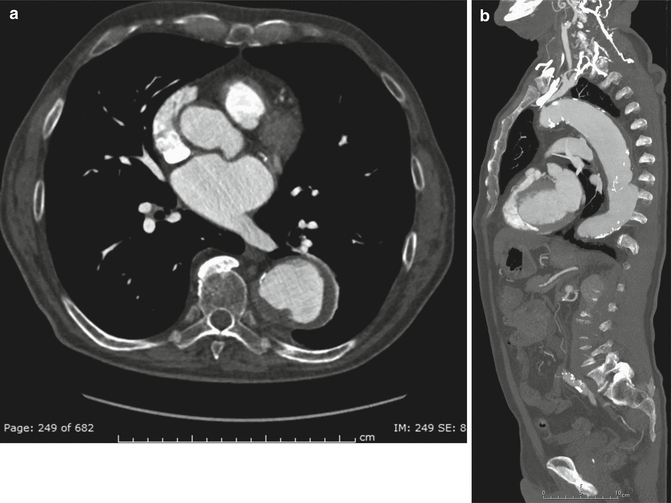

Fig. 4
Descending TAA in an 82-year-old man. Contrast-enhanced (a) axial CT image shows an atherosclerotic aorta, with fusiform aneurysm. (b) Sagittal MIP image shows the overall extent of the aneurysm in the descending aorta
Because an abdominal aortic aneurysm occurs in 28 % of patients with TAA, initial CT evaluation may include the entire thoracoabdominal aorta (Bickerstaff et al. 1982).
Many authors consider genetic causes as a leading factors in the formation and development of aortic aneurysms. Inherited disorders of connective tissue contribute to the formation of aortic aneurysms. Marfan syndrome is a multisystemic connective tissue, autosomal dominant inherited disorder (Goldstein et al. 2015). One of the hallmark features is dilatation or dissection of the aortic root (Daimon et al. 2008). Aortic aneurysms without annuloaortic ectasia are also common. Annuloaortic ectasia, especially with dilatation of the aortic root, is found in 60–80 % of adults with Marfan syndrome. It usually begins with dilatation of the aortic sinuses, which progresses first into the sinotubular junction, than into the aortic annulus. Dilatation of the aortic root causes aortic valve insufficiency that may progress to aortic root dissection or rupture (Vasan et al. 1995). The aortic aneurysms rarely show intimal calcification or atherosclerotic thrombosis; they occur commonly and develop more rapidly in younger patients. After the initial diagnosis of aortopathy by TTE, CT or MRI is recommended to confirm the size of the aorta and to document the diameters of the distal ascending aorta, aortic arch, and descending aortic segments. Repeated CT or MRI, about every 3 years, is recommended to reassess the aortic arch and the descending aorta. Prophylactic surgery for annuloaortic ectasia is recommended when the diameter of the Valsalva sinus exceeds 5.5 cm in an adult and 5.0 cm in a child, when the diameter of the Valsalva sinus is less than 5.0 cm but in a rapid rate of aortic aneurysm expansion (increase in dilatation by more than 1 cm per year), or when there is a family history of aortic dissection (Wolak et al. 2008; Kälsch et al. 2013; De Backer et al. 2006). Composite surgical replacement of the aortic root and valve is commonly performed with or without coronary reimplantation (Fig. 5a, b).
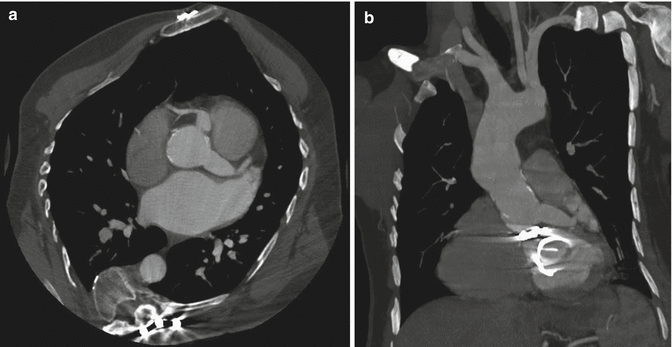

Fig. 5
Axial–oblique (a) and coronal–oblique MIP (b) CT sections at the level of the coronary artery origin, after ascending aorta replacement with graft. Note the persistent dilation of both proximal left and right coronary arteries
Other genetic conditions associated with aortic dilatation are bicuspid valve-related aortopathy (BAV), Loeys–Dietz syndrome, Turner syndrome, familial TAA, and Ehlers–Danlos syndrome. Ehlers–Danlos syndrome (EDS) is an autosomal dominant disorder with vascular involvement (arterial dilatation and rupture). The imaging characteristics of aortic aneurysms in Ehlers–Danlos syndrome resemble those in Marfan syndrome.
Aortitis is a general term that refers to a broad category of infectious or noninfectious conditions, in which there is abnormal inflammation of the aortic wall. These inflammatory conditions have different clinical and morphologic features and variable prognoses (Restrepo et al. 2011). The differentiation between infectious (mycotic) and noninfectious aneurysms is important because, as mycotic aneurysms are treated with antibiotic therapy and surgical debridement with or without surgical revascularization, noninfectious aneurysms are managed by steroids and immunosuppressive drugs (Nagpal et al. 2015).
Noninfectious aortitis may be part of a systemic disorder (Gornik and Creager 2008). The association between rheumatic diseases and aortic involvement is well known, but the prevalence of aortic involvement in the different rheumatic diseases is quite variable. Rheumatic diseases (Slobodin et al. 2006) with a high prevalence (>10 %) of aortic involvement include Takayasu arteritis (Fig. 6a–c), giant cell arteritis (GCA), long-standing ankylosing spondylitis, Cogan syndrome (interstitial keratitis, iritis, conjunctival or subconjunctival hemorrhage, fever, aortic insufficiency) (Fig. 7a, b), and relapsing polychondritis. Rheumatic diseases in which aortic involvement is an uncommon well-documented complication include rheumatoid arthritis, seronegative spondyloarthropathies, Behçet disease, and systemic lupus erythematosus (SLE). Aortitis most commonly affects the ascending aorta in rheumatoid arthritis (Fig. 8a, b), ankylosing spondylitis, giant cell arteritis, and relapsing polychondritis (Posniak et al. 1990). These conditions may also be associated with aortic valve insufficiency. Aortitis in rheumatic fever can be segmental, limited to the ascending aorta, or it can involve the abdominal aorta or the entire aorta (Lande and Berkmen 1976; Posniak et al. 1990). Takayasu arteritis commonly affects the aortic arch and its major branches, with variable involvement of the abdominal aorta and pulmonary arteries; although Takayasu arteritis typically causes arterial stenosis and occlusion, aneurysms may also occur. Inflammatory aneurysms differ from atherosclerotic aneurysms because of the presence of dense perianeurysmal fibrosis and thickened aortic wall (Restrepo et al. 2011). The prevalence is 5–25 % of all abdominal aortic aneurysms. Inflammatory aneurysms of the ascending aorta and aortic arch are much less frequent, usually associated with concomitant inflammatory aneurysms in the abdominal aorta (Girardi and Coselli 1997). CT shows a hypoattenuating mass with periaortic wall thickening that spares the posterior wall. After intravenous administration of contrast material, rapid luminal opacification is followed by delayed enhancement of the soft-tissue component.
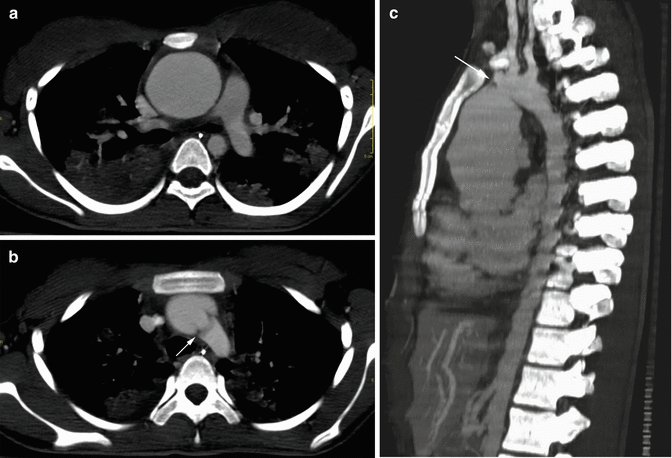
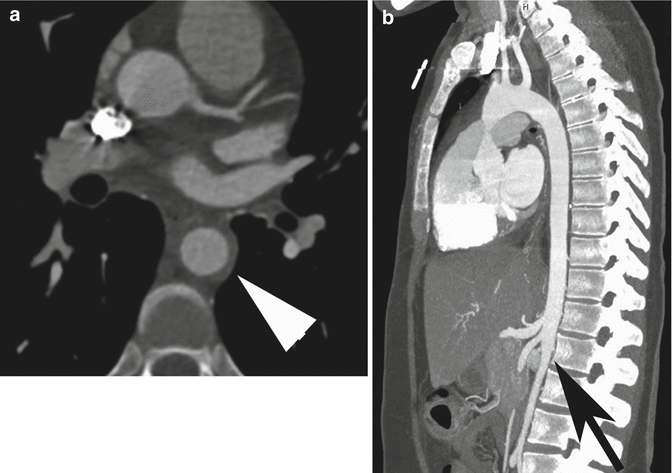
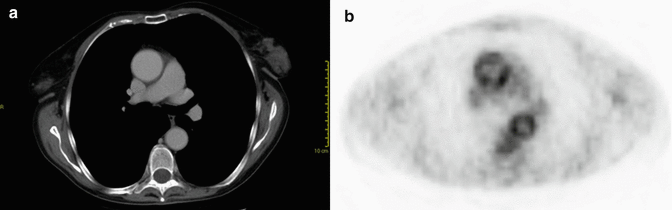

Fig. 6
Axial (a, b) and sagittal (c) CT MIP images in a patient with Takayasu arteritis. Fusiform aneurysm of the ascending aorta (a, c) and atypical coarctation at the level of the aortic arch (arrows in b, c) are well demonstrated

Fig. 7
Axial (a) and sagittal MIP (b) CT images of a patient with Cogan syndrome, showing circumferential wall thickening of the normal caliber descending aorta (arrow head in a) and abrupt narrowing of the abdominal aorta distal to the mesenteric artery origin (arrow in b)

Fig. 8
Axial CT (a) and PET (b) images of a patient with rheumatoid arteritis. There is questionable wall thickening of the normal caliber thoracic aorta at CT (a). PET shows abnormal uptake of the aortic wall due to the inflammatory changes
Infected (mycotic) aneurysms. Infectious aortitis is secondary to gram-positive bacteria such as the Staphylococcal species, Enterococcus species, and Streptococcus pneumonia, responsible of 60 % of the infections. Gram-negative bacilli (Salmonella species in the majority of cases) are also a frequent cause of aortic infection; however, they are more prevalent in infectious abdominal aortitis (Revest et al. 2007). Aortic infection by Mycobacterium tuberculosis, an uncommon problem in developed countries, may occur as a result of the extension to the aortic wall of a contiguous infective focus such as infected mediastinal lymph nodes or lung lesions. Treponema pallidum is a rare etiology today, with a historical importance. Aortic infection by unusual bacteria and nonbacterial (fungi) microorganisms is extremely rare; however, this possibility must be considered in immunocompromised patients (Gornik and Creager 2008). Infected (mycotic) aneurysms are uncommon, and they usually involve segments not commonly involved by atherosclerosis (Lee et al. 2008). Although the infraabdominal aorta is the most frequently involved segment of the aorta, there is also a combined involvement of the descending thoracic, thoracoabdominal, and suprarenal aorta (Oderich et al. 2001). Early changes of aortitis preceding aneurysm formation include an irregular arterial wall, periaortic edema as fat stranding or a hypoattenuating concentric rim at CT, a periaortic soft-tissue mass, and periaortic gas. Concentric or eccentric periaortic inflammatory soft tissue can develop, as a homogeneous contrast-enhancing mass at CT (Ting and Cheng 1997; Tsao et al. 2002; Azizi et al. 2004). The inflammatory mass can develop necrosis with a heterogeneous attenuation at CT, with rim enhancement or poor enhancement after administration of contrast material (Gomes and Choyke 1992). Periaortic mass and fat stranding are the most common imaging findings of infected aortic aneurysms, and they are found in 48 % of cases (Macedo et al. 2004). Periaortic gas is an uncommon feature (Vogelzang and Sohaey 1988; Macedo et al. 2004). At CT, an infected aortic aneurysm appears as a focal, contrast-enhancing dilatation, usually saccular. The lumen can be central or eccentric and it can be a single compartment or multiloculated. Disrupted arterial wall calcifications can occur adjacent to the infected aneurysm (Sueyoshi et al. 1998; Azizi et al. 2004). Calcification within the aneurysmal wall and thrombus within an infected aneurysm are uncommon (Gomes and Choyke 1992). An infected aneurysm can rapidly develop and enlarge (Sueyoshi et al. 1998; Macedo et al. 2004), and subsequently it can rupture due to high systemic arterial pressure.
Posttraumatic aneurysms or, better, pseudoaneurysms, following blunt trauma may result from rapid deceleration, which is the most accepted mechanism of injury (Agarwal et al. 2009), with shearing and bending stress on the aortic wall. According to this theory, during the sudden deceleration, the distal transverse arch moves forward while the proximal descending thoracic aorta remains stationary, held back by the ligamentum arteriosum and the intercostal vessels (Javadpour et al. 2002). Another proposed mechanism is the “osseous pinch,” where an anteroposterior compression force results in posteroinferior displacement of the manubrium, first rib, and medial clavicle, which impinge on the aorta and compress it against the thoracic spine posteriorly (Crass et al. 1990). A third possible mechanism of aortic injury is represented by the sudden increase of intraluminal blood pressure due to trauma with the so-called water hammer effect (Creasy et al. 1997). The most common site of injury, seen in survived trauma victims, is the aortic isthmus (90 % of cases), followed by the ascending aorta and the descending aorta near the diaphragmatic hiatus (Mukherjee and Rajagopalan 2007). Chronic pseudoaneurysms develop in 2.5 % of patients who survive the initial trauma. These often calcify (Fig. 9), may contain thrombus (Heystraten et al. 1986), and have the potential to enlarge progressively, rupturing even years after the initial trauma (Posniak et al. 1990).
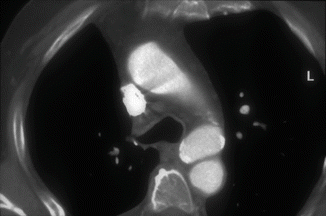

Fig. 9
Axial CT in a patient with chronic posttraumatic pseudoaneurysm of the aortic isthmus. There is no mediastinal hematoma and there are some calcifications along the aneurysmal wall
Aortic dissection is an abnormal passage of blood, through an intimal tear, into the media, producing a false lumen separated from the true lumen by an intimal flap. A previous aortic dissection, with a persistent false channel, may produce aneurysmal dilatation of the false lumen. These are false aneurysms, contained only by the outer media and adventitia, enlarging over time (Fig. 10).
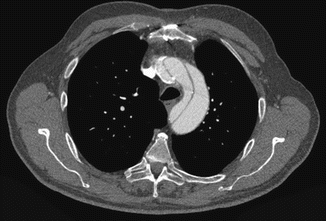

Fig. 10
Axial CT image at the aortic arch level in a patient with chronic dissection. The dilated aortic arch with intimal flap, false and true lumens are well demonstrated
1.5 Pitfalls
Normal aortic variants can mimic an aortic aneurysm. Three of these are ductus diverticulum, Kommerell’s diverticulum, and aortic spindle.
1.5.1 Ductus Diverticulum
Ductus diverticulum consists of a convex focal bulge along the anterior undersurface of the isthmic region of the aortic arch (Gotway and Dawn 2003). Although ductus diverticulum is commonly believed to be a remnant of the closed ductus arteriosus, it has been suggested that this entity may actually represent a remnant of the right dorsal aortic root (Grollman 1989). It is particularly important to differentiate ductus diverticulum from a posttraumatic aortic pseudoaneurysm, which most commonly occurs at the aortic isthmus. In contrast to a pseudoaneurysm, ductus diverticulum has smooth margins with gently sloping symmetric shoulders and forms obtuse angles with the aortic wall (Gotway and Dawn 2003).
1.5.2 Aortic Spindle
Aortic spindle is a smooth, circumferential bulge located below the isthmus, in the first portion of the descending aorta. It should not be confused with an aneurysm (Agarwal et al. 2009) (Fig. 11a–c).
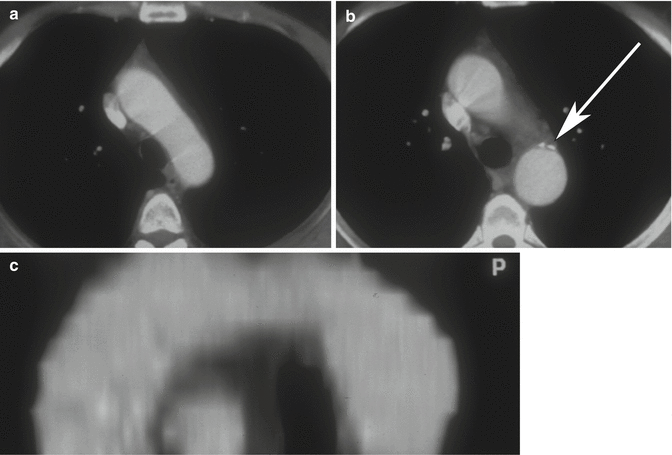

Fig. 11
Axial CT images (a, b) and sagittal–oblique MPR image (c) of aortic spindle, which is a normal mild dilation of the most proximal descending thoracic aorta. Note the calcific densities at the level of ductus arteriosus insertion (arrow)
1.5.3 Kommerell’s Diverticulum
Right aortic arch (RAA) is an uncommon anatomical variant, occurring in about 0.1 % of the population (Shuford et al. 1986). Two main types are commonly seen: mirror-image branching (type I), commonly associated with congenital cyanotic heart disease, and aberrant left subclavian artery (LSA) (type II) (Yeo et al. 2015). Type II RAA is often accompanied by a Kommerell’s diverticulum, an aneurismal diverticulum that develops at the origin of the LSA and the proximal aspect of descending aorta (Edwards 1948). Because of the atherosclerotic changes occurring in the arterial wall during life, in adults it is generally not possible to distinguish a true diverticulum (Kommerell’s diverticulum, an embryonic remnant) from an acquired aneurysm of the origin of the aberrant LSA. Patients with type II RAA are often asymptomatic; they are diagnosed incidentally in adulthood or when complications arise from compression of the mediastinal structures, caused by a growing Kommerell’s diverticulum (Hastreiter et al. 1966). However, it was originally described as a diverticular outpouching at the origin of an aberrant right subclavian artery with a left-sided aortic arch (Fig. 12a, b).
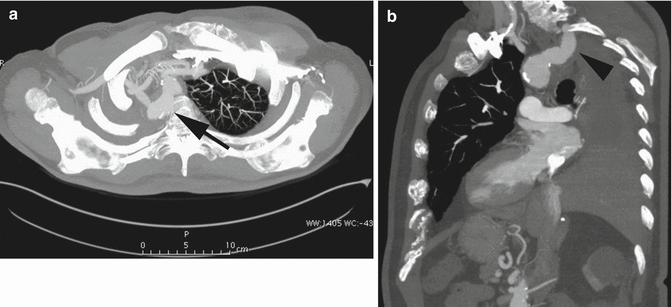

Fig. 12
Axial (a) and sagittal oblique (b) CT MIP images in a patient post-right pneumonectomy, with retroesophageal right aberrant subclavian artery with proximal aneurysmal dilation: Kommerell’s diverticulum (arrow in a, arrowhead in b)
1.6 Complications
1.6.1 Rupture
The risk of rupture of TAAs increases with the size of the aneurysm (Gotway and Dawn 2003).
Indications for surgical treatment are summarized in the Guidelines of the American Heart Association (Hiratzka et al. 2010):
Aortic size – ascending aortic diameter ≥5.5 cm or twice the diameter of the normal contiguous aorta; descending aortic diameter ≥6.5 cm; subtract 0.5 cm from the cutoff measurements if the following are present: Marfan syndrome, family history of aneurysm or connective tissue disorder, bicuspid aortic valve, aortic stenosis, dissection, and patient undergoing another cardiac operation; growth rate ≥1 cm/year
Symptomatic aneurysm
Traumatic aortic rupture
Acute type B aortic dissection with associated rupture, leak, distal ischemia
Pseudoaneurysm
Large saccular aneurysm
Mycotic aneurysm
Aortic coarctation
Bronchial compression by aneurysm
Aortobronchial or aortoesophageal fistula
Contraindications are always individualized on patient’s ability to undergo a major surgery. High-risk patients and elderly persons must be always considered for less invasive procedures. Patients with end-stage renal disease, respiratory insufficiency, cirrhosis, or other major comorbid conditions are the most critical.
CT is the modality of choice for identifying aneurysmal rupture. CT findings of aneurysm rupture. Thoracic aortic aneurysms can rupture into the mediastinum (Fig. 13a, b), pleural cavity, pericardium, or adjacent luminal structures such as the airways or the esophagus. CT findings are a high-attenuation hematoma on nonenhanced scans and an active contrast material extravasation from the aortic lumen on contrast-enhanced scans (Agarwal et al. 2009). An important imaging feature, in a contained rupture of TAA, is the “draped aorta sign”: it occurs when the posterior wall of the aorta is not identifiable as distinct from adjacent structures or when it closely follows the contour of adjacent vertebral bodies (Halliday and al-Kutoubi 1996). Imaging features suggestive of instability or impending rupture include increased aneurysmal size, a low thrombus-to-lumen ratio, and hemorrhage into a mural thrombus (Rakita et al. 2007). Nonruptured aneurysms generally contain more thrombus than ruptured aneurysms and thrombus-to-lumen ratio decreases with the increase of the aneurysmal size (Pillari et al. 1988). It is intuitive that a thick circumferential thrombus is protective against rupture and an enlargement of the patent lumen is indicative of partial lysis of the thrombus, which predisposes an aneurysm to rupture (Pillari et al. 1988; Mower et al. 1997). A focal discontinuity in the circumferential wall calcifications (Fig. 14) is more commonly observed in unstable or ruptured aneurysms (Siegel et al. 1994); this finding is really important when, in comparison with a previous CT, the area of discontinuity in mural calcifications is new. A well-defined peripheral crescent of increased attenuation (Fig. 15a, b) within the thrombus of a large TAA is a CT sign of acute or impending rupture (Mehard et al. 1994; Gonsalves 1999). This finding is best appreciated on unenhanced CT images. It represents an internal dissection of blood into the peripheral thrombus or into the aneurysm wall, when the ability of the thrombus to protect the aneurysm from rupture is shrinking (Arita et al. 1997). It is one of the earliest and most specific CT signs of the rupture process (Mehard et al. 1994; Siegel et al. 1994; Arita et al. 1997; Gonsalves 1999). A TAA can develop fistulous communication with the tracheobronchial tree and the esophagus. The aortobronchial fistula manifests clinically as hemoptysis (Lesko et al. 1997) and at CT as consolidation in the adjacent lung due to hemorrhage; the fistulous communication cannot be easily seen at CT (Coblentz and Sallee 1988). Most aortobronchial fistulas (90 %) occur between the descending aorta and the left lung (MacIntosh et al. 1991). Aortoesophageal fistula is less common. It manifests clinically as hematemesis and dysphagia (Cho et al. 2004) and at CT as mediastinal hematoma, as an intimate relationship of the aneurysm to the esophagus, and, rarely, as contrast material extravasation into the esophagus (Green and Klein 2007).
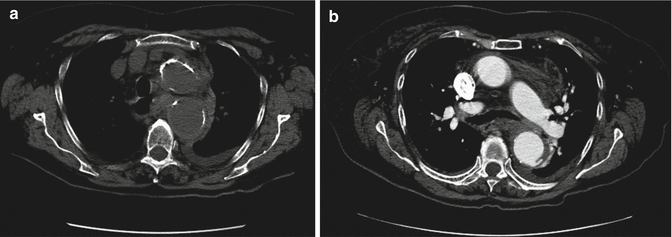
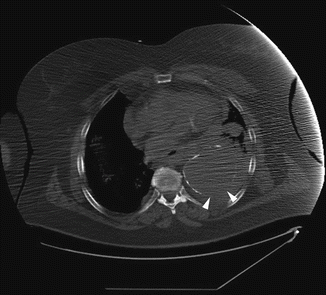
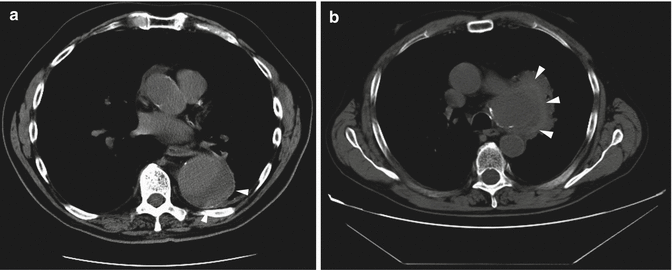

Fig. 13
Unenhanced (a) and enhanced (b) axial CT scan of a patient with ruptured aortic aneurysm. Unenhanced image (a) shows discontinuity in wall calcifications, high-attenuation hematoma, and draping of the posterior aortic wall. Enhanced image (b) shows active extravasation of contrast material into the thrombus. Both images demonstrate blood into mediastinum and left pleural cavity

Fig. 14
Unenhanced axial CT sections of a patient with contained rupture of aneurysmal descending aorta. The discontinuity of wall calcifications is clearly shown on the posterior and lateral side of aorta (arrow heads)

Fig. 15
Unenhanced axial CT scan of two different patients with hemorrhage into a mural thrombus. A peripheral crescent-shaped area of hyperattenuation (arrow heads in a, b), corresponding to the hematoma into the mural thrombus, is displayed in (a) a patient with impending rupture of an aneurysmal descending aorta with thrombus and (b) in a patient with contained rupture of aortic arch aneurysm into the mediastinum (b)
1.6.2 Compression of Adjacent Structures
TAAs can produce symptoms by compressing adjacent structures, such as superior vena cava syndrome due to compression of the superior vena cava, stridor or dyspnea due to airway compression, hoarseness due to compression of the recurrent laryngeal nerve, and dysphagia due to esophageal compression (Posniak et al. 1990).
1.7 Clinical Presentation
Thoracic aortic aneurysms often grow slowly and usually without symptoms, making them difficult to detect. TAAs are usually found incidentally after chest radiographs or during other imaging studies. Most patients are hypertensive or can have a history of vascular disease in different districts, such as coronary or carotid arteries, but, in general, they remain relatively asymptomatic until the aneurysm expands.
The most common symptom is pain. Chest or back pain in presence of TAA is predictive of aortic rupture. Ascending aortic aneurysms tend to cause anterior chest pain, whereas arch aneurysms more likely cause pain radiating to the neck. Descending thoracic aneurysms more likely cause back pain localized between the scapulae. When located at the level of the diaphragmatic hiatus, the pain occurs in the mid-back and epigastric region; however, identifying the area of aortic involvement on the basis of the specific location of pain is not always reliable and can be misleading. Large ascending aortic aneurysms can cause superior vena cava obstruction with distended neck veins. Ascending aortic aneurysms also may develop aortic insufficiency and heart failure. Arch aneurysms, where stretching of the recurrent laryngeal nerves is present, may cause hoarseness. Descending thoracic aneurysms and thoracoabdominal aneurysms may compress the trachea and/or bronchi and cause dyspnea, stridor, wheezing, or cough. Compression of the esophagus results in dysphagia. They can also erode the surrounding structures and result in aortobronchial or aortoesophageal fistulas with, respectively, hemoptysis, hematemesis, or gastrointestinal bleeding. Erosion into the spine causes back pain or instability. Spinal cord compression or thrombosis of spinal arteries may result in paraparesis or paraplegia. Descending thoracic aneurysms may thrombose or embolize clot and atheromatous debris distally to visceral, renal, or lower extremity arteries.
1.8 CT Technique and CT Data Manipulation
In the clinical setting of acute nontraumatic chest pain, case history, physical examination, ECG, and laboratory risk markers should be immediately obtained. Chest X-ray is useful to rule out many nonvascular causes of acute chest pain such as pleural effusions, pneumothorax, etc. In the field of nontraumatic vascular emergencies, CT has a primary role with a sensitivity and a specificity near 100 % for acute aortic pathologies, while MRI is indicated mainly for follow-up of aortic pathologies due to longer scan time, limited availability, and increased cost (Nagpal et al. 2015).
The CT protocol starts with unenhanced scan to visualize intramural hematoma that can be misdiagnosed as atherosclerotic thrombus in the arterial phase CT scan (Chiu et al. 2013). The scan should be extended above the aortic arch (for aortic branches) and caudally to the femoral heads to cover aorta entirely. Then contrast-enhanced CT with high infusion rate (4–5 mL/s) is performed to achieve a target opacification of the aorta >250 HU, obtained in the arterial phase with a test bolus or a bolus-tracking technique. The contrast should be injected through the right upper extremity to avoid artifacts from left innominate vein in the area of the aortic arch. A saline flush of 30–40 mL should be injected following contrast medium to minimize streak artifact from superior vena cava (Weininger et al. 2011). Delayed images are recommended after stent–graft repair of aortic aneurysm to detect endoleaks (Task et al. 2014). ECG gating when available is very useful to avoid cardiac motion artifacts impairing the evaluation of thoracic aorta and coronary origin. ECG-gated imaging both in precontrast and in postcontrast CT, from above aortic arch to diaphragmatic hiatus, should be included in the standard protocols for thoracic vascular emergency. Prospective ECG gating is performed at mid-diastole if heart rate is <70 beats per minute, while it is acquired in end-systole in patients with heart rate >70 beats per minute (Abbas et al. 2014). High-quality images of aortic root and proximal coronary tracts can be obtained without ECG gating using newer CT scanners (128 slice CT) with fast gantry rotation time and iterative reconstruction algorithm to reduce both X-ray dose and contrast media volume (Russo et al. 2015).
MDCT images are initially evaluated in axial sections, but two-dimensional and three-dimensional reformatting techniques such as multiplanar reformation (MPR), maximum intensity projection (MIP), and virtual rendering (VR) allow better diagnosis of aortic pathology. In particular to obtain correct measurement of the aortic diameters, it is necessary to use MPR images perpendicular to the centerline of the aortic lumen to avoid overestimation (Fig. 16a). Measurements of the aortic lumen on the transaxial CT images are incorrect when the central axis of the aorta is not perpendicular but oblique to the transaxial CT image (Fig. 16b).
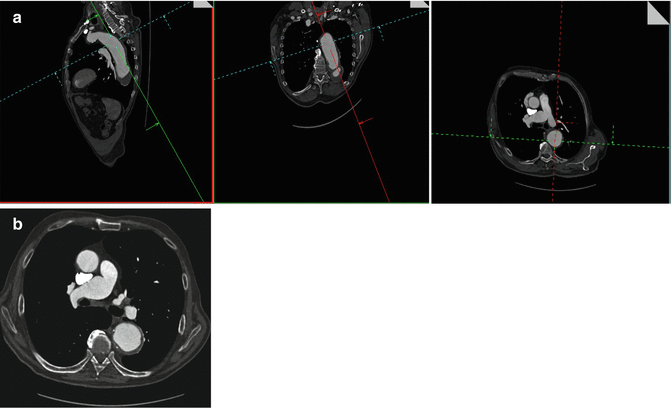

Fig. 16
A 77-year-old woman with descending aortic aneurysm. Multiplanar postcontrast CT images show the correct (a) technique of wall-to-wall measurement of the descending aortic aneurysm perpendicular to its center line. Pure axial postcontrast CT image of the same patient shows the incorrect (b) measurement technique of the aneurysm due to overestimation
MIP is a useful tool to display vascular map (Fig. 17) but doesn’t demonstrate the 3D relationship with nonvascular structures, while volume rendering (VR) allows to display vascular anatomy and provides, at the same time, definition of the surrounding structures (Fishman et al. 2006) (Fig. 18). It is the only way to transfer to the referring clinician the 3D perspective of the aortic aneurysm.
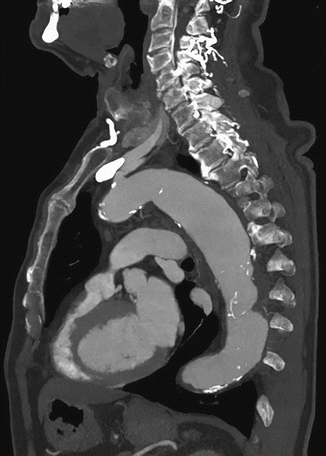
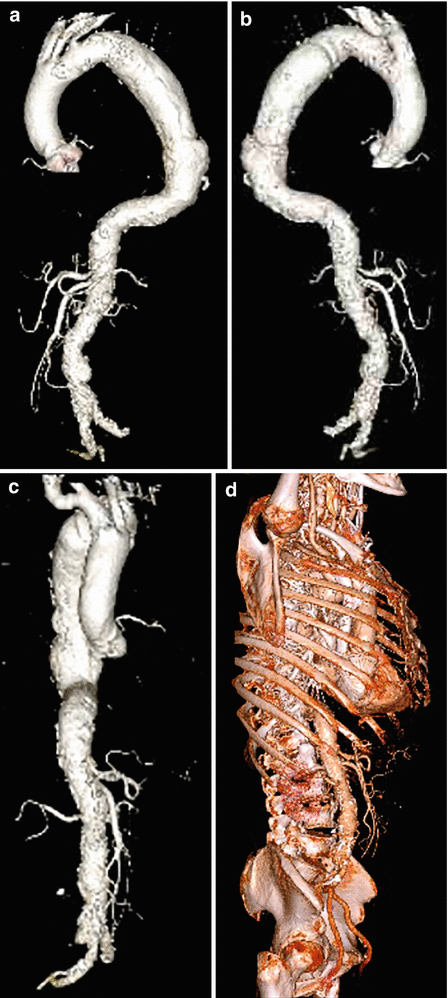

Fig. 17
MPR sagittal view with MIP CT image of thoracic aorta aneurysm

Fig. 18
3D volume-rendered CT images of thoracic aorta aneurysm in lateral (a, b) and frontal view (c). In (d) 3D volume-rendered CT image of the same patient without bone elimination
A standard report describing thoracic aorta should include the following measurements (Agarwal et al. 2009):
Sinus
Sinoaortic junction
Midascending aorta
Proximal aortic arch
Midaortic arch
Proximal descending aorta
Aorta at diaphragm
Every report of an aneurysmal thoracic aorta must include the relationships between the aneurysm and the aortic branches, to assess the possibility of endovascular repair. Minimum length of 15 mm from aortic lesion and left subclavian artery/celiac trunk and maximum aortic landing zone diameter of 40 mm without circumferential thrombus within the landing zone are essential to perform endovascular procedure (Garzón et al. 2005).
2 Thoracic Aortic Thrombosis
2.1 Introduction
With the advent of recently developed imaging techniques and the routine exploration using TEE after any embolic events, the presence of thrombi in the aorta has been increasingly identified. The primary source of peripheral arterial embolism (PAE) is cardiac in more than 85 % of cases (Reber et al. 1999). Among noncardiac sources, the aorta has been reported in up to 5 % of cases to be the origin of PAE (Gagliardi et al. 1988).
Thrombus formation can be found both in atheromatous and in apparently normal aorta.
2.2 Definition, Etiology, and Clinical Presentation
Aortic mural thrombi usually arise as a consequence of preexisting aortic pathology. Thrombogenic lesions such as aneurysm, complex atherosclerotic plaque with protruding atheromas, and/or dissection are found to underlie the vast majority of aortic thrombi (Rossi et al. 2002).
Common well-recognized embolic sources of aortic thrombosis include intracardiac thrombus or myxoma.
Thrombi in the thoracic aorta are even much less common, particularly in apparently normal aorta. It has been initially associated to premature atherosclerosis, as a result of prior trauma, but some authors suggested as a separate clinical entity.
Primary aortic mural thrombus (PAMT) is defined as a thrombus formation in a morphologically normal aorta without any evidence of cardiac source.
In 1958, H. Gaylis described the first case of PAMT: the thrombus was located in the abdominal aorta (Gaylis 1958). Later, Machleder et al. confirmed this clinical entity: in 10,671 consecutive autopsies, he found 95 cases of mural thrombus of the thoracoabdominal aorta and reported an incidence of 0.45 % (48 cases) of nonaneurysmal aortic mural thrombus, with 17 % of these patients having evidence of distal embolization (Machleder et al. 1986).
Because the disease is mostly asymptomatic, the true incidence of PAMT remains unknown. In two large series that evaluated patient with PAE, the incidence reported was higher and varies from 5 to 20 % (Verma et al. 2014).
Verma et al. identified 19 PAMT in a retrospective study of 88 patients who underwent extensive evaluation of acute occlusion of extremities or visceral arteries. Mural thrombus was located in the thoracic aorta in ten patients (52 %) and in the abdominal aorta in nine (48 %) (Verma et al. 2014).
The most frequent thoracic location of PAMT is the region of the aortic isthmus and the portion distal to the aortic arch, at the side opposite to the origin of the subclavian artery (Choukroun et al. 2002). Also, localization of aortic thrombus in the ascending aorta has been described (Pousios et al. 2009).
PAMT is more frequent in young women, and it has been associated in patients with essential thrombocytosis (Fang et al. 2001) and Crohn’s disease (Lehmann et al. 2001). Smoking, steroid therapy, elevated fibrinogen levels and/or hypercoagulability, and numerous underlying pathologies have been reported.
Underlying pathologies described in morphologically normal thoracic aortic thrombosis
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|