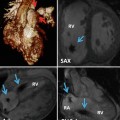
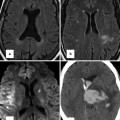
Fig. 14.1
(a) Upper row: comparison between mitral (m) and tricuspid (t) valves in normal heart versus atrioventricular septal defect (AVSD). Two types of AVSDs including common and partial (separate valves) are shown. Note the membranous septum (MS) position in normal heart and its absence in AVSD. Also note unwedged position of the aorta in AVSD. Lower row: different forms of shunting in AVSD are shown. In partial AVSD, only a septum primum atrial septal defect (ASD) exists. In complete form, both ASD and ventricular septal defect (VSD) usually exist. (b) Complete form of AVSD, Rastelli type A. p pulmonary valve, LA left atrium, RA right atrium, MS Membranous septum, LV left ventricle, RV right ventricle
Both morphological types have the following pathological features:
1.
Alteration of the fibrous skeleton of the heart characterized by the replacement of mitral and tricuspid fibrous rings by a common fibrous atrioventricular annulus. Trifoliate appearance of the left atrioventricular valve (cleft).
2.
Absence of membranous septum and midline fibrous continuity between the left and right leaflets of the common valve. Fibrous continuity with aortic annulus may remain.
3.
Unwedging of the aortic root.
4.
A ventricular septal defect comprising perimembranous and inlet areas.
5.
A relatively short ventricular inlet with an elongated outlet causing increased apex-aortic sigmoid distance (gooseneck). Inlet-outlet disproportion is more pronounced in complete form.
6.
Excavated (scooped out) appearance of the ventricular septum which is more obvious in complete form.
7.
Superior-inferior arrangement of left ventricle papillary muscles.
8.
Posteroinferior displacement of the AV node.
9.
Increased rate of the left ventricular outflow tract narrowing due to elongation of the outflow tract and adherence of the anterosuperior bridging leaflet to the scooped ventricular septum.
The interatrial septum is the least affected anatomy. In a large number of hearts, the interatrial septum has normal dimensions with no septal defect [13–15]. There are cases with AVSDs, in which the septal structures are intact and exhibit pathological stigmata of this malformation [16, 17].
An important feature in the diagnosis of AVSD is the type of insertion and the degree of extension of the anterosuperior bridging leaflet, which constitutes the basis for Rastelli type A, B, and C classification. Intermediate forms of insertion between A and B and between B and C and mixed forms have been described. In AVSD, when atrioventricular valves are removed, it is almost impossible to distinguish between the two basic types [8].
In the morphological diagnosis of AVSD, it is important to pay attention to associated anomalies in the hearts including the presence of a common atrium caused by absence of interatrial septation, the parachute insertions of part of the tensor apparatus of the common AV valve, tetralogy of Fallot with double outlet right ventricle [18], and the presence of double valve orifices both in half right and in half left of the common AV valve.
In the embryonic heart, the AV septum arises from growth, development, and fusion of the dorsal and ventral mesenchymal endocardial cushions of the AV canal, which divides it into two AV canals: left and right. On the cephalic part of the left AV canal, the annulus of the mitral valve is differentiated, and at the bottom of the right AV canal, the annulus of the tricuspid valve will be formed. The AV septum so formed has two ends; the cephalic end is associated with the transient foramen primum and the caudal end is related to interventricular septal defect. These two septal defects will be obliterated by fusion of the cephalic and caudal ends of the AV septum to the interatrial septal complex and the ventricular septum, respectively. The cephalic end of AV septum also bends to the left and differentiates into the central portion of the medial valve or anterior leaflet of the mitral valve. Many authors agree that AVSD originates by the lack of growth, development, and fusion of the dorsal and ventral endocardial cushions of the embryonic atrioventricular canal [12, 19]. There is also lack of growth of the ventricular septum and membranous septum causing inlet and perimembranous interventricular septal defect. These two facts explain all pathological stigmata which present the AVSD.
Imaging Techniques
Echocardiography has become the modality of choice for the complete assessment of AVSDs [20]. This technique includes all of the standard views to optimize imaging of specific structures. The subcostal views allow to determine abdominal and atrial situs. The long-axis view will determine the cardiac position within the thorax. Long- and short-axis views show the systemic venous and some of the pulmonary venous return and the size and location of any atrial septal defects. Both the right and left ventricular outflow tracts can be evaluated from these projections. A slow sweep in the short-axis view toward the apex will reveal the size and location of ventricular level shunts, the cross-sectional anatomy of the AV valves, and the location and number of left ventricular papillary muscles. This view is also good for the evaluation of ventricular function. From the short-axis plane, the transducer is rotated counterclockwise approximately 45º to assess AV valve morphology and attachments to the ventricular septum and papillary muscle anatomy. It is the best view to evaluate if the valve tissue is balanced over the two ventricles. The atrial and ventricular septal defects are best seen from the apical four-chamber view. A slow sweep from the apical window offers good visualization of the leaflet anatomy, AV valve attachments, defect size and location, and some information on the mechanism of AV valve regurgitation. This view is also used to measure ventricular length. The left ventricular outflow tract is imaged from the apical two-chamber view. Careful imaging of mitral valve attachments to the ventricular septum, in addition to color and spectral Doppler interrogation, is used to evaluate the presence of, and potential for, left ventricular outflow tract obstruction. Parasternal long-axis imaging gives another excellent view of the anatomy of the left ventricular outflow tract.
High parasternal imaging will complete the assessment of the branch pulmonary arteries and pulmonary venous return and the presence of patent ductus arteriosus and a left superior vena cava. The aortic arch should be imaged either from a high parasternal window or from the suprasternal notch. Long-axis imaging is used to evaluate for aortic arch obstruction. Short-axis views are used to determine aortic arch sidedness and the branching pattern of the brachiocephalic arteries and to exclude a vascular ring [15, 20].
The echocardiography provides precise images of the anatomic findings of this congenital malformation with details of the AVSD, the common AV valve or two separated AV valves, the relationships between the leaflets of the valves in both forms, septal structures and their defects, disproportion between the left ventricular inflow and outflow tracts, unwedged aorta, and the shunts that determine the clinical presentation. This anatomic information is of enormous value for the clinician in planning surgical treatment.
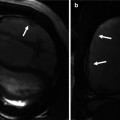
Recent studies demonstrate the incremental value of three-dimensional transthoracic echocardiography (3DTTE) over two-dimensional transthoracic echocardiography (2DTTE) in the evaluation of the AVSDs. Because the various morphological features of AVSDs could be viewed in three dimensions, a more comprehensive and accurate assessment of the size and extent of the septal defects; size, number, and abnormalities of AV valve leaflets (Fig. 14.2) and their attachment sites; as well as the relation of the valvular structures to the great vessels is provided by 3DTTE. In some cases, 3DTTE served to verify the findings seen on 2DTTE and, therefore, increased the confidence level with which various morphological features or abnormalities were detected. In others, 3DTTE substantially changes the morphological diagnosis by providing additional details not apparent on 2DTTE. The three-dimensional datasets also provide en face views of defects in the AV valves such as commissures, resulting in more accurate assessment of their size and extent. Such views are especially helpful when planning for surgical repair.
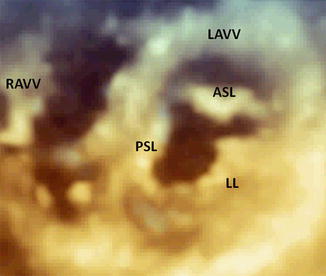

Fig. 14.2
Three-dimensional transthoracic echocardiography of an AVSD with two valves, showing a trifoliated atrioventricular structure in diastole. RAVV right atrioventricular valve, LAVV left atrioventricular valve, PSL posterior septal leaflet, ASL anterior septal leaflet, LL lateral leaflet
Color Doppler is useful not only in evaluating the three-dimensional extent of valvular regurgitant jets in the atrial chambers but also in providing en face views of the valve orifices, facilitating assessment of its exact size. This has the potential of providing a more accurate quantitative assessment of regurgitant volumes as compared to 2DTTE methods [15, 21, 22].
Echocardiography is limited, however, in the evaluation of certain portions of the aorta (particularly the ascending aorta and the transverse arch), the distal pulmonary arteries, the right ventricle, and the pulmonary veins. These are relative “blind spots” for the echocardiographer. MRI, unhindered by “blind spots,” is excellent for the depiction of cardiac anatomy. In all cases of AVSD, the tricuspid and mitral valves originate at the same level. These findings are best seen on axial or four-chamber MR images. Cine MRI can demonstrate the presence of atrial and ventricular septal defects and valve regurgitation.
Cine MR sequences are used to obtain multiple images over the cardiac cycle at each anatomic location. Spin echo pulse sequences are usually used to produce images in which blood signal intensity is low and appears dark (“black blood” imaging). An ECG-gated velocity-encoded cine MRI (VEC MRI) sequence, a type of gradient echo sequence, can be used to measure blood flow velocity and quantify blood flow rate [23].
Multislice CT has important strengths in comparison with each of these imaging modalities, because it provides excellent anatomic information [24]. The primary disadvantage of CT is that it requires the use of ionizing radiation. Echocardiography, MRI, and CT are complementary in diagnosis of AVSD. Intracardiac anatomy is well depicted by all three modalities. Function and flow quantification are well assessed with Echocardiography and MRI. CT provides exquisite images of the great vessels and associated extracardiac anomalies [25].
Anatomo-Imaging Correlation
Anatomo-imaging correlation between heart specimens with AVSD and echocardiogram, MRI, and CT studies of patients with equivalent findings has demonstrated a high degree of precision that can be achieved with noninvasive imaging diagnosis [1].
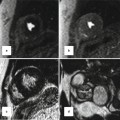
The AVSDs can be found in any situs of the heart, situs solitus and situs inversus (Fig. 14.3), or in the isomeric positions of dextroisomerism or levoisomerism (Fig. 14.4). In all four situs positions, AVSD can occur in its two forms of common valve (Fig. 14.4) or two separate valves (Fig. 14.5). It is the relationships among the leaflets within the common AV junction that determine the number of AV orifices, while the relationships between the leaflets and the septal structures dictate the possible shunts that can be established between the two sides of the heart [14–17, 26]. Other anatomic characteristics that can be determined by noninvasive imaging include the interatrial or interventricular septal defects. Failure of closure of the ostium primum results in a large atrial septal defect, although in some cases the ostium primum is absent and the interatrial septum is complete [27]. The integrity of the atrial septum can be examined with a 4-chamber image at the level of the AV junction and the inferior portion of the atrial septum (Fig. 14.6). If the septum is complete, its inferior portion reaches the AV junction and there is no ostium primum defect (Fig. 14.6b, c). When the atrial septum is deficient, its inferior portion is far above the plane of the AV junction and an ostium primum defect is present. Cases have been reported in the literature in which there are more than one atrial septal defect [14–16]. In those cases in which the atrial septum is fused with the leaflets of the common AV valve, a shunt would only be possible through a ventricular septal defect. Two cases are reported in the literature in which the septal defects were obliterated by fusion of the AV valve leaflets to the cardiac septa, and the patients had no septal defects [16, 17].
Get Clinical Tree app for offline access

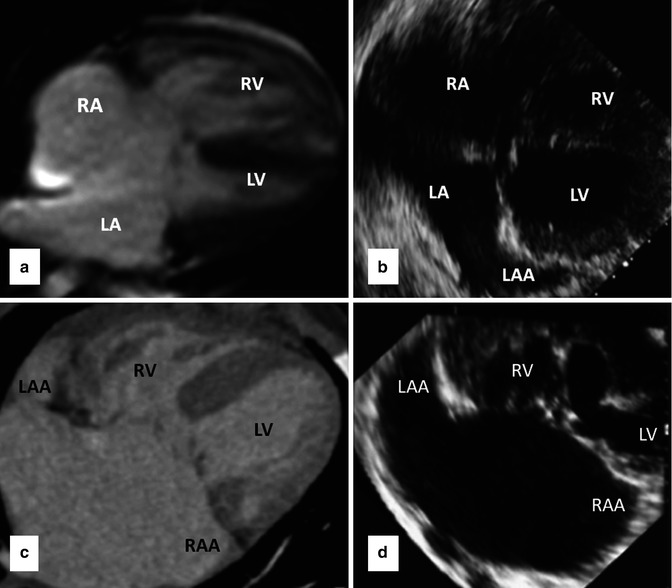
Fig. 14.3
Upper row images are four-chamber cine MRI (a) and transthoracic echocardiography (b) in AVSD with a common valve in atrial situs solitus. Lower row




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree