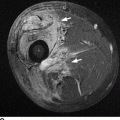
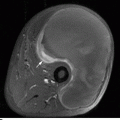
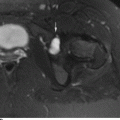
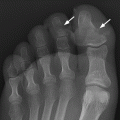
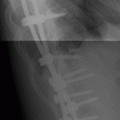
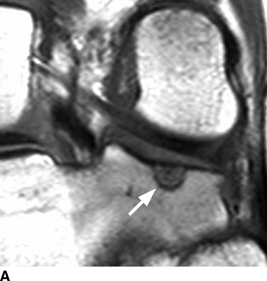
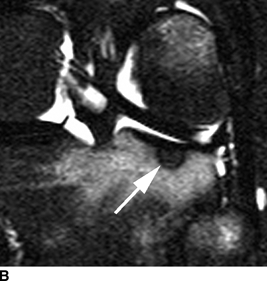
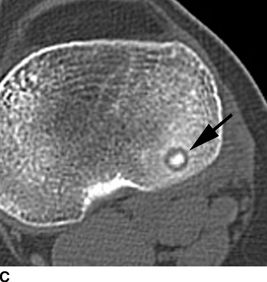
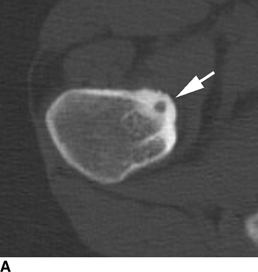
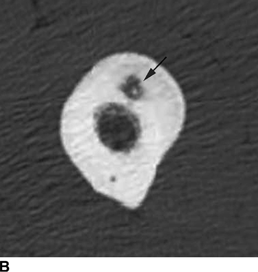
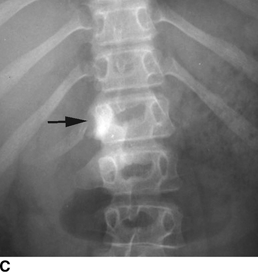
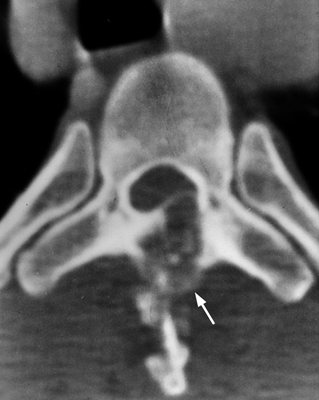
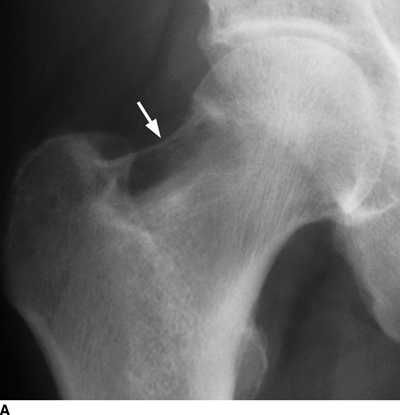
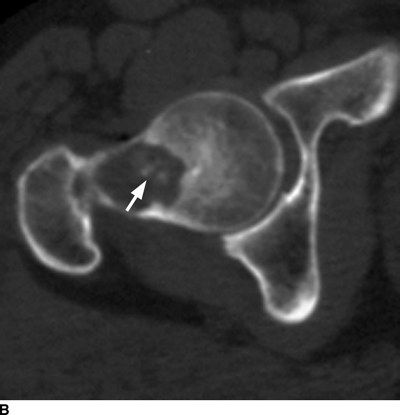
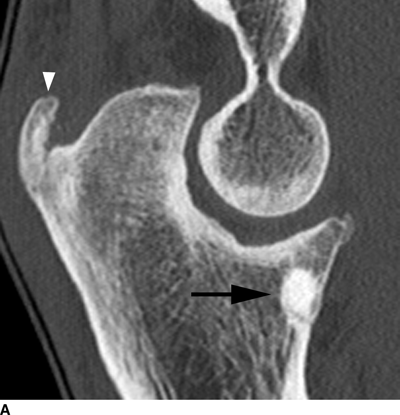
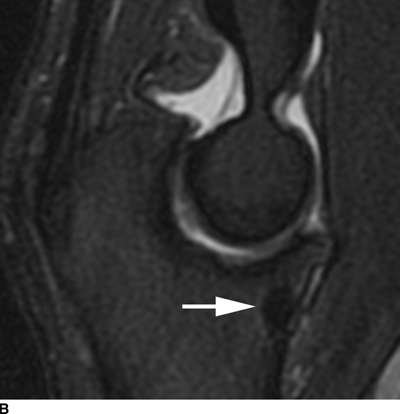
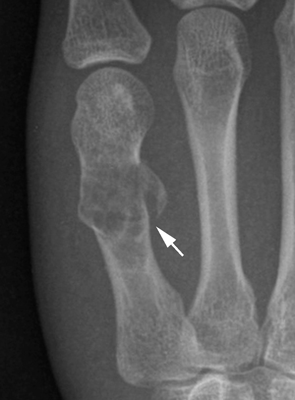
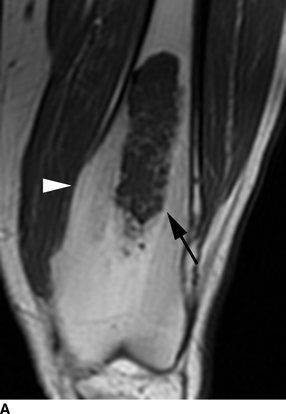
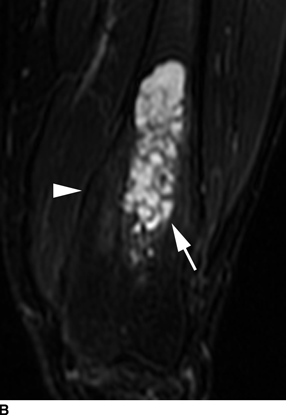
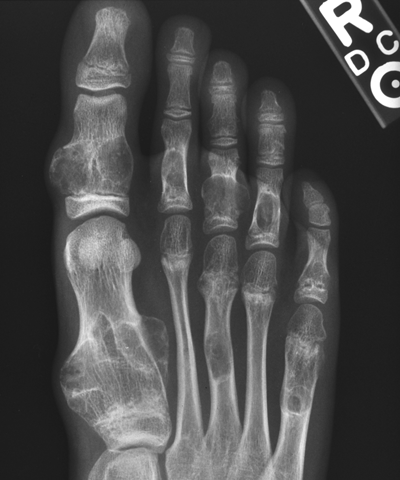
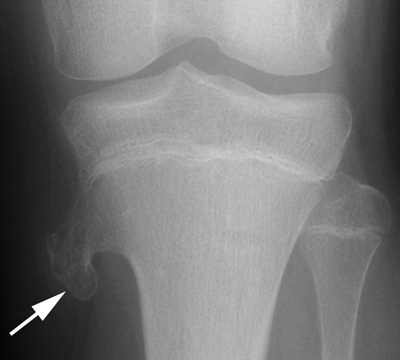
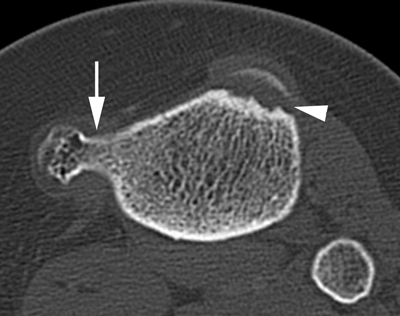
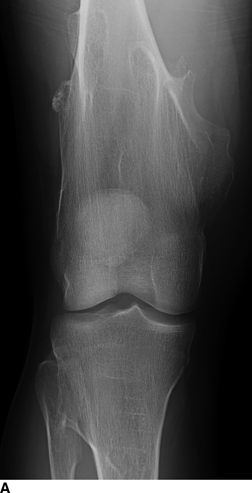
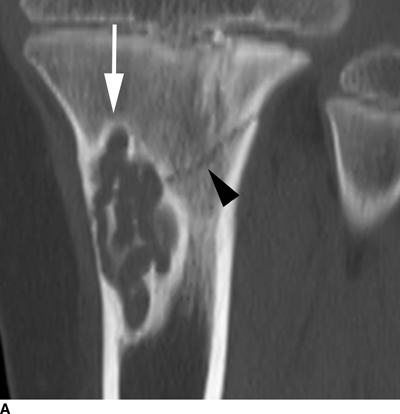
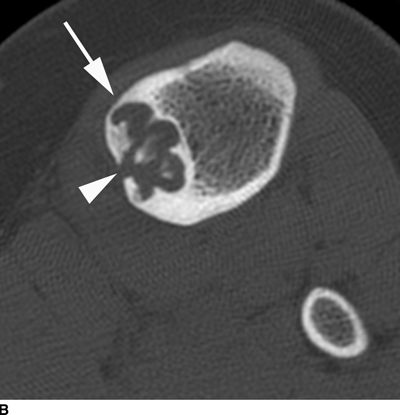
CHAPTER 9 This chapter describes benign neoplasms and benign tumorlike lesions that are not neoplastic in origin. A key decision that becomes easier with experience is whether a radiologic finding represents an actual lesion or merely a normal structure, an anatomic or developmental variant, or the result of previous trauma, surgery, or other disease. Atlases of normal variants are often helpful. The principal lesions one should consider in formulating a differential diagnosis for a benign lesion are listed in Table 9.1. TABLE 9.1 Benign Bone Lesions aThere may be case reports of multiple lesions. Osteoid osteoma is a fairly common benign bone-forming neoplasm. The lesion, called a nidus, is small, generally ranging in size from 3 to 15 mm. When larger, the lesion is often classified as an osteoblastoma (discussed in “Osteoblastoma”). Approximately 80% of patients are between the ages of 5 and 20 years; few are older than 30 years of age. The location may be cortical, central, or, less frequently, subperiosteal. The femur is the most common site (39% of cases), followed by the tibia (23%) and upper extremity (21%). Patients present with dull, aching pain that has lasted for months to years. When located in the spine, osteoid osteomas may cause painful scoliosis of rapid onset; near a joint, they may cause reactive synovitis. The nidus consists of osteoid and fibrovascular connective tissue. The osteoid may be mineralized to a variable extent, usually in the center (Fig. 9.1). The nidus is radiolucent but may be obscured by cortical thickening and dense sclerosis from the reactive bone. Sclerosis is usually minimal around lesions located in the cancellous bone, near a joint, or in a subperiosteal position (Fig. 9.2). Osteoid osteomas have intensely increased activity on bone scan; the activity may be more intense in the nidus (“double-density” sign). The nidus may enhance on CT and MRI, reflecting the fibrovascular tissue within it. Because of the small size of the nidus and its possible obscuration by reactive sclerosis, CT is commonly used to identify and localize the nidus. Osteoid osteomas have been treated by surgical excision, but their small size makes them amenable to percutaneous radiofrequency ablation or excision under CT guidance. Failure to remove or destroy the entire nidus may lead to recurrence. FIGURE 9.1. Osteoid osteoma. A: Coronal T1-weighted MRI shows lesion in the subchondral bone of the lateral tibial plateau. B: Coronal T2-weighted fat-suppressed MRI shows the nidus (arrow) with surrounding edema. C: Axial CT scan shows central ossification within the nidus (arrow). FIGURE 9.2. Osteoid osteoma (different patients). A: Axial CT scan shows a round nidus (arrow) in the lesser trochanter with moderate reactive bone formation. B: Axial CT scan shows a round nidus with central calcification (arrow) in the cortex of the femoral shaft, with exuberant reactive sclerosis. C: AP radiograph shows reactive scoliosis with an osteoid osteoma in the T12 vertebra (arrow). Osteoblastomas are uncommon lesions that are considered by some to be giant osteoid osteomas because of their histologic resemblance. They affect young persons; 80% occur in patients younger than 30 years of age. Approximately half are found in the spine and most of the remainder in the femur and tibia. Of those in the spine, most are in the posterior elements; a few also involve the vertebral body, and very few involve the vertebral body alone. Within tubular bones, osteoblastomas may be intracortical, subperiosteal, or central in location. They are larger than osteoid osteomas, generally 1 to 10 cm in diameter. There is usually much less reactive bone surrounding an osteoblastoma than an osteoid osteoma. Patients with osteoblastomas typically present with pain of several months’ duration. If the lesion is superficial, localized swelling and tenderness are present. In the spine, lesions may produce painful scoliosis; they may also interfere mechanically with the cord or with nerve roots. The radiographic appearance is a partially lucent expansile lesion with well-defined margins and a variable amount of matrix mineralization (Figs. 9.3 and 9.4; see also Fig. 7.5). The lucent area of geographic bone destruction corresponds to replacement of the bone by nonmineralized tumor tissue. The tumor osteoid may have densely solid or ground-glass mineralization. Large lesions may have an expanded cortical shell from slow endosteal cortical erosion balanced against an enlarging layer of periosteal new bone. Cortical penetration into the soft tissues is absent, but tomography may be required to demonstrate the cortical shell. The tumor tissue may have high attenuation on CT from diffuse mineralization. Osteoblastomas are hot on bone scan. Osteoblastomas usually grow slowly and respond well to excision; very few have been reported to become locally aggressive. FIGURE 9.3. Osteoblastoma. CT shows an expansile lesion in the neural arch of a lower thoracic vertebra (arrow). FIGURE 9.4. Osteoblastoma. A: Radiograph shows a lucent lesion (arrow) in the femoral neck with a sclerotic margin. B: CT shows partial mineralization (arrow). A bone island (enostosis) is a circular or oblong nodule of cortical-type bone lying within cancellous bone. Bone islands are typically 1 cm in size but range up to 4 cm. They consist of histologically normal compact bone with haversian systems. They may be found anywhere in the skeleton. Radiographically, they are homogeneous and are as dense as cortical bone. They are well defined but have peripheral spiculations that merge with the surrounding trabeculae (Fig. 9.5). Occasionally, they may change in size or remodel. On radionuclide bone scan, isotope uptake is absent or minimal. Bone islands are common and large or giant bone islands may be mistaken for other lesions such as osteoblastic tumors; they have no other clinical significance. Small bone islands are common incidental findings on CT and MRI. FIGURE 9.5. Bone island. A: Bone island in the cancellous bone of the coronoid process of the elbow (arrow) on a sagittal reformatted CT. The margins are spiculated where they blend into the normal cancellous bone. The lesion is as dense as the cortex. There is an incidental olecranon enthesophyte (arrowhead). B: Sagittal T2-weighted fat-suppressed MRI shows the bone island to have low signal intensity (arrow). Solitary enchondromas are benign neoplasms located within the medullary cavity that are composed of mature hyaline cartilage. They probably arise from cartilaginous rests displaced from the growth plate. Sex incidence is equal, and most patients are between 10 and 50 years of age. Typically, the lesions are asymptomatic and discovered incidentally, but many patients present with pathologic fractures. The most common locations for solitary enchondromas are the hands (approximately 50% of cases), the proximal and distal femur, and the proximal humerus. In the hands, the middle and distal portions of the metacarpals and the proximal portions of the phalanges are typically involved. Radiographically, these lesions are lucent from replacement of the bone by the nonmineralized cartilage, but the typical mineralization patterns of cartilaginous matrix may be present: dense punctate or flocculent calcifications or ring- or arc-shaped densities from enchondral ossification of lobular cartilage (Fig. 9.6; see also Figs. 7.12 and 7.13). Slow endosteal enlargement causes an expanded, thinned cortex, but cortical penetration is absent. On MRI, enchondromas have low signal on T1-weighted and proton density images but high signal on T2-weighted images with foci of low signal intensity corresponding to areas of calcification. A characteristic lobular configuration is virtually diagnostic (Fig. 9.7). On radionuclide bone scan, increased isotope uptake is seen, reflecting enchondral ossification, hyperemia, and reactive bone formation. FIGURE 9.6. Enchondroma with lucent matrix occupying the midshaft of the fifth metacarpal.The overlying cortex is thinned. A pathologic fracture is present (arrow). FIGURE 9.7. Enchondroma. A: Coronal T1-weighted MRI shows low signal and lobular configuration (arrow). Deformity of the distal femoral diaphysis (arrowhead) is due to a sessile osteochondroma. B: Coronal inversion recovery MRI shows high signal in the lesion (arrow) and low signal in the osteochondroma (arrowhead). Occasionally, it may be difficult to distinguish an enchondroma from a low-grade chondrosarcoma radiographically. Imaging findings that suggest a chondrosarcoma include deep endosteal scalloping (greater than two thirds of cortical thickness), cortical destruction and soft-tissue mass (at CT or MRI), periosteal reaction (at radiography), marked uptake of radionuclide (greater than the anterior iliac crest) at bone scintigraphy, and destruction of chondroid matrix over time. Development of chondrosarcoma from solitary enchondroma has not been proved conclusively. Multiple enchondromatosis (Ollier disease) is a nonfamilial, nonheritable diffuse growth abnormality in which the tubular bones may be bowed and shortened to a variable extent and filled with multiple enchondromas (Fig. 9.8). The severity of involvement may range from a few lesions with mild deformities to countless lesions with severe deformities. The lesions often become stable at puberty, but their growth may continue throughout life. Individual lesions are radiologically and histologically identical to solitary enchondromas, but patients with multiple enchondromatosis have a 30% to 50% risk of developing chondrosarcoma. A lesion that becomes painful in the absence of pathologic fracture should trigger consideration of malignancy. Multiple enchondromatosis with multiple soft-tissue hemangiomas is called Maffucci syndrome. The hemangiomatosis may be localized or extensive and may occur anywhere in the skin or subcutaneous tissues. FIGURE 9.8. Multiple enchondromatosis (Ollier disease) deforming the foot.Some lesions have calcified matrix. Osteochondromas (also called benign exostoses) are outgrowths of a histologically normal bone that arise in the vicinity of a growth plate. They are exceedingly common and present during late childhood and adolescence. Although any bone with enchondral ossification may be involved, the femur, the proximal tibia, and the proximal humerus account for two thirds of cases. The typical site is metaphyseal, but osteochondromas may arise anywhere near a growth plate, including accessory ossification centers. Osteochondromas are not thought to be neoplastic but result from the growth of aberrant foci of cartilage on the bony surface. As the cartilage grows, it forms a cap over a bony mass that develops by progressive enchondral ossification. The bony portion contains mature, normal cortical and medullary bone with a marrow space contiguous with the parent bone. Deep lobules of cartilage may be present, often heavily ossified. The lesion may be pedunculated on a stalk, sometimes resembling a cauliflower (Figs. 9.9 and 9.10). Osteochondromas may also be sessile and resemble an expansile lesion. The cartilage cap largely disappears by adulthood. The typical clinical presentation is a firm mass of long duration that may mechanically interfere with function. A bursa may form over the surface and produce pain; in the presence of hemorrhage in a bursa, an enlarging mass develops. FIGURE 9.9. edunculated osteochondroma (arrow) arising from the medial tibial metaphysis. FIGURE 9.10. Axial CT scan of osteochondroma showing its marrow space to be contiguous with that of the underlying bone (arrow). The open tibial tubercle growth plate is visible anteriorly (arrowhead). Osteochondromatosis (multiple heritable exostoses, multiple osteochondromas, and diaphyseal aclasis) is one of the most common skeletal dysplasias. The condition is familial, with more severe manifestations in men. The skeleton is involved symmetrically, and the limbs are affected more than the spine. The number of exostoses varies. Deformities of the tubular bones are present (Fig. 9.11) and cause disproportionately short limbs, but the degree of shortness appears to be unrelated to the number of exostoses. Growth of the lesions slows as the skeleton matures, and new lesions do not appear in adulthood. Multiple exostoses are radiologically and histologically indistinguishable from solitary exostoses. Secondary development of chondrosarcoma in a solitary exostosis or in one of the multiple exostoses is a small but definite risk, probably on the order of 1% to 2%; the onset of pain or growth in an adult suggests the possibility. The radiologic distinction between a benign exostosis and an exostotic chondrosarcoma is difficult unless growth and change in appearance can be demonstrated on serial films. FIGURE 9.11. Multiple hereditary osteochondromatosis. A, B: Radiographs of the knee and shoulder show multiple bony exostoses and deformity of the tubular bones. Periosteal chondroma is a benign neoplasm composed of a mature cartilage that is located beneath the periosteum. The cortical surface is eroded, but the medullary cavity is not involved. Most are located in the metaphysis or diaphysis of a long bone; the humerus is the most common site. The lesions are lucent and are characteristically surrounded by smoothly contoured, solid, periosteal reactive bone (Fig. 9.12). Cartilage matrix calcification may be present. Symptoms are nonspecific; local excision is curative. FIGURE 9.12. Periosteal chondroma of proximal humerus. Chondroblastoma (Codman tumor) is an uncommon benign neoplasm that consists of chondroid tissue mixed with more cellular tissues. Location in the epiphysis is characteristic (approximately 98%), often with extension into the metaphysis (Fig. 9.13). Two thirds arise in the lower extremities, and half arise around the knee. Most patients are young; 80% are between 5 and 25 years of age. When chondroblastomas occur outside the usual age group, they arise in unusual locations. The presentation is nonspecific, typically pain. The radiographic appearance is an ovoid or rounded lucent epiphyseal lesion that is eccentrically located. The margins are geographic, usually with a thin, reactive bony rim. Scattered mottled or stippled calcifications, like those of other cartilage tumors, may be present. Chondroblastomas treated by curettage usually do not recur, but some are aggressive locally. FIGURE 9.13. Chondroblastoma on axial CT shows a lucent lesion (arrow) with a sclerotic margin and cartilaginous matrix in the talus. Chondromyxoid fibromas are rare, cartilage-forming neoplasms with variable amounts of myxoid and fibrous tissue. Young adults are affected most often, but there is a broad age range. The presentation is usually pain. Most chondromyxoid fibromas are found in the lower extremities. The typical radiographic appearance is an eccentric metaphyseal lucent lesion, ovoid or round and lobulated, with a sclerotic rim (Fig. 9.14). Intralesional matrix mineralization is rare. Sometimes, the rim has thickly trabeculated walls, corresponding to a grossly lobular tumor. Cortical expansion may result in a thin, perhaps imperceptible, cortical shell. The treatment is curettage. FIGURE 9.14. Chondromyxoid fibroma. A: Axial CT scan shows a lucent lesion with chondroid matrix mineralization in the ischium (arrow). B: Axial T2-weighted fat-suppressed MRI showing high signal within the lesion (arrow). Fibrous cortical defects and nonossifying fibromas (fibroxanthomas) are histologically identical nonneoplastic lesions thought to be the result of faulty ossification at the growth plate. A causal relationship with stress or trauma has been suggested but not proved. These are self-limited, with no potential for growth or spread. Both regress spontaneously, filling in with bone from the periphery and disappearing. The lesions are present at some time in perhaps one third of all children. Fibrous cortical defects (metaphyseal fibrous defects) are seen most typically in children aged 4 to 8 years. They are located on the cortical surface of the metaphysis at the attachment of a tendon or ligament, mostly around the knee, and produce a 1- to 4-cm scalloped defect in the underlying bone. They are round or oval, lucent, and sharply marginated by a sclerotic rim (Fig. 9.15). Some have a bubbly appearance. Pathologic fractures may occur, but fibrous cortical defects are usually clinically silent and disappear within 2 years of discovery. Nonossifying fibromas can be thought of as fibrous cortical defects with somewhat different radiographic appearance; the distinction is not important for the patient. They are less common than fibrous cortical defects and are discovered in older children or adolescents. They are located eccentrically within the medullary cavity but still within an expanded cortex and are lucent with a sclerotic margin. Some lesions have a trabeculated, scalloped, multilocular, or bubbly appearance (Figs. 9.16 and 9.17; see also Fig. 7.4). The size range is 1 to 7 cm, and larger lesions may fracture or cause pain. These lesions show a variable amount of uptake on 18F-FDG PET imaging and may mimic metastatic disease, thus making review of radiographs, CT, or MRI of utmost importance to evaluate for the typical imaging appearance of these benign lesions. FIGURE 9.15. Fibrous cortical defect with lobulated sclerotic border (arrow) and pathologic fracture (arrowhead). A: Coronal reformatted CT. B: Axial CT.
Benign Lesions
 BENIGN BONE-FORMING LESIONS
BENIGN BONE-FORMING LESIONS
Osteoid Osteoma
Osteoblastoma
Bone Island
 BENIGN CARTILAGE LESIONS
BENIGN CARTILAGE LESIONS
Enchondroma
Osteochondroma
Osteochondromatosis
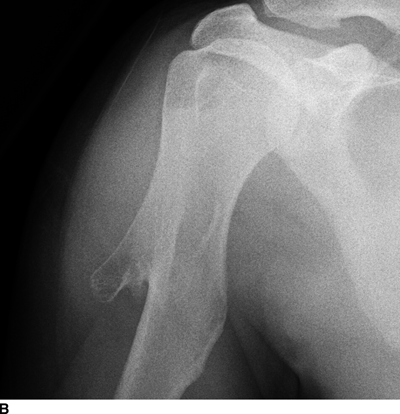
Periosteal Chondroma
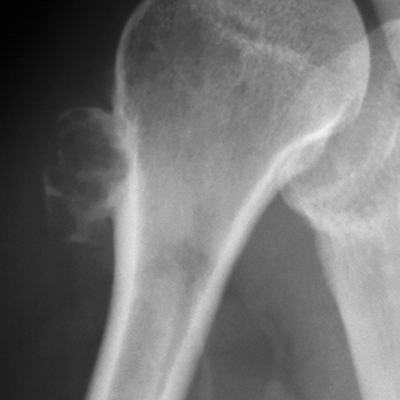
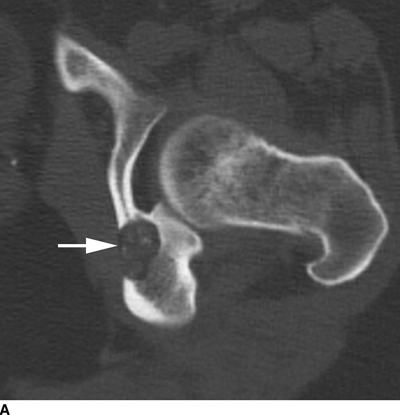
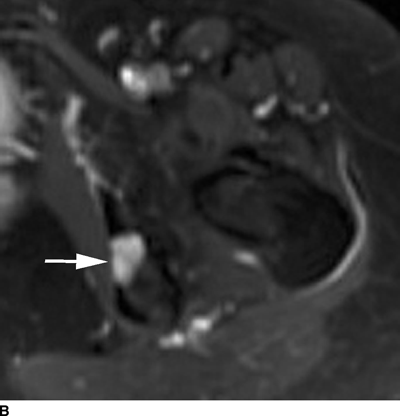
Chondroblastoma
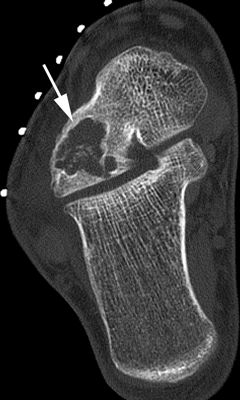
Chondromyxoid Fibroma
 BENIGN FIBROUS LESIONS
BENIGN FIBROUS LESIONS
Fibrous Cortical Defects and Nonossifying Fibromas
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree