Benign Tumors and Tumor-like Lesions IV: Miscellaneous Lesions
Simple Bone Cyst
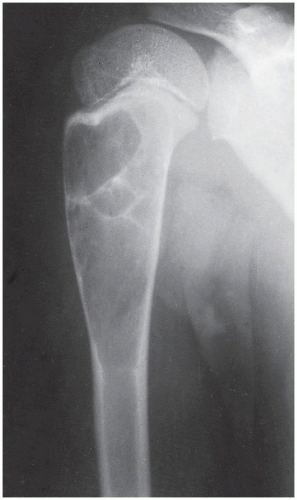
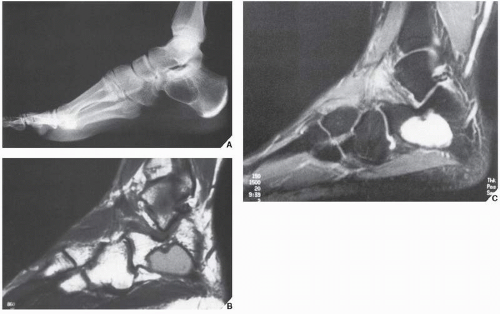
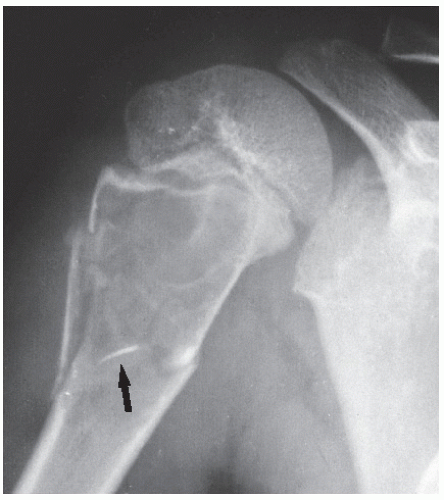
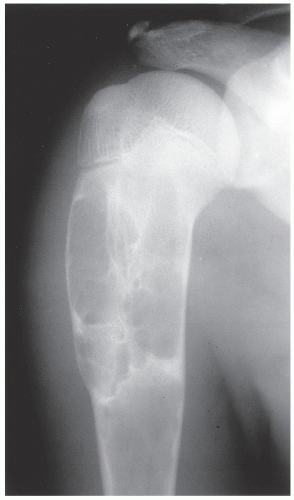
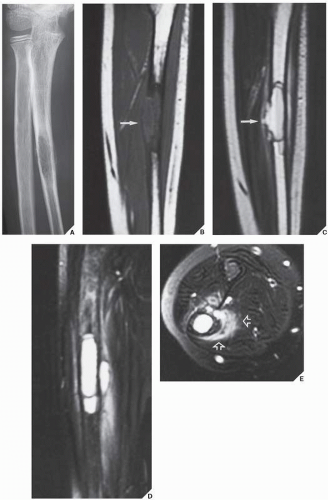
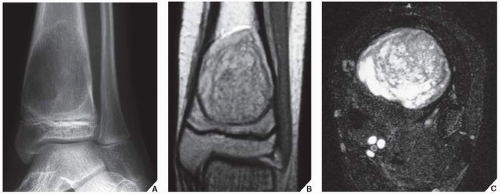
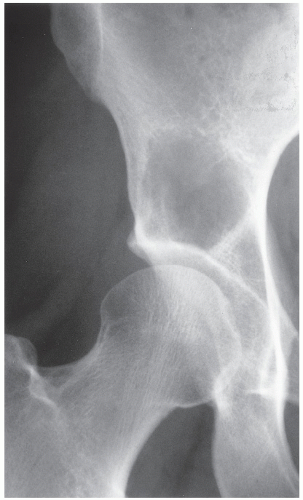
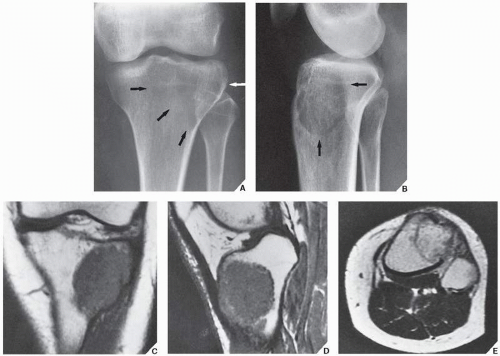
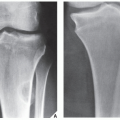
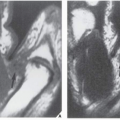
The simple bone cyst (SBC), also called a unicameral bone cyst, is a tumor-like lesion of unknown cause, representing approximately 3% of all primary bone lesions. It has been attributed to a local disturbance of bone growth. Although the pathogenesis is still unclear, SBC appears to be reactive or developmental rather than represent a true neoplasm. More common in males than in females, it is ordinarily seen during the first two decades of life. The majority of SBCs are located in the proximal diaphysis of the humerus and femur, especially in patients younger than age 17 years. In older patients, the incidence of bone cysts in atypical sites such as the calcaneus, talus, and ilium increases significantly (Fig. 20.1). The clinical symptoms include pain, swelling, or stiffness at the nearest joint. A pathologic fracture is often the first sign of the lesion. Radiographically, SBC appears as a radiolucent, centrally located, well-circumscribed lesion with sclerotic margins (Figs. 20.2,20.3,20.4,20.5). There is no periosteal reaction, a feature distinguishing a SBC from an aneurysmal bone cyst (ABC), which invariably shows some degree of periosteal response; however, in the presence of pathologic fracture, there is periosteal reaction. Conventional radiography usually suffices to make a diagnosis. Magnetic resonance imaging (MRI) of SBC shows the signal characteristics of fluid: a low-to-intermediate signal on T1-weighted images and a bright, homogeneous signal on T2 weighting (Fig. 20.6).
Histologically, SBC is a diagnosis of exclusion. A surgical curettage yields almost no solid tissue, but the walls of the cavity may show remnants of fibrous tissue or a flattened single-cell lining. The fluid content of the cyst contains elevated levels of alkaline phosphatase.
Complications and Differential Diagnosis
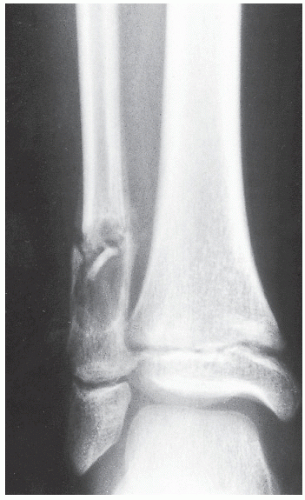
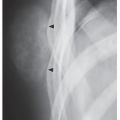
The most common complication of SBC is pathologic fracture, which occurs in approximately 66% of cases (Fig. 20.7). Occasionally, one can identify a piece of fractured cortex in the interior of the lesion— the “fallen fragment” sign—indicating that the lesion is either hollow or fluid filled, as most SBCs are. This sign permits the differentiation of a bone cyst, particularly in a slender bone, such as the fibula (Fig. 20.8), from other radiolucent, radiographically similar lesions containing solid fibrous or cartilaginous tissue, such as fibrous dysplasia, nonossifying fibroma, or enchondroma (Fig. 20.9).
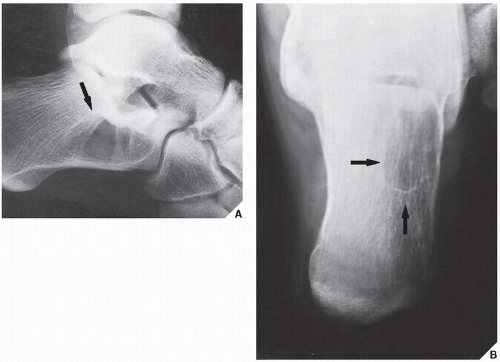
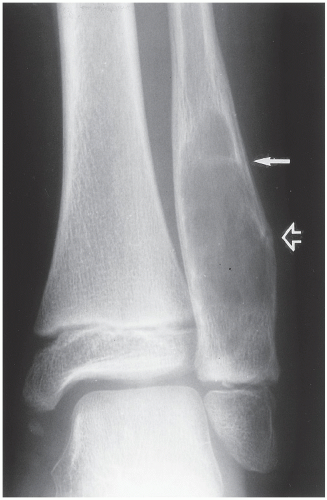
A bone abscess may occasionally mimic a SBC, particularly if located in the proximal humerus or proximal femur, the sites of predilection for SBCs. In such cases, the presence of a periosteal reaction and extension beyond the growth plate are important differentiating features favoring a bone abscess (Fig. 20.10). On rare occasions, an intraosseous ganglion may be mistaken for a SBC (Fig. 20.11).
Treatment
The treatment of SBCs is based on the premise that the induction of osteogenesis results in complete healing of the lesion. The simplest inducement for bone repair is fracture, but this alone is insufficient to obliterate the lesion completely, and SBCs usually do not disappear after spontaneous fracture. The most common treatment is curettage followed by grafting with small pieces of cancellous bone. With this procedure, however, there is a higher rate of recurrence in patients younger than age 10 years. Moreover, this approach may lead to damage to the growth plate, because most solitary bone cysts occur about the physis. Recently, Scaglietti reported treating bone cysts with simple injection of methylprednisolone acetate. In younger patients so treated, complete bone repair occurred more rapidly than in older patients, who sometimes had to be administered several injections.
Aneurysmal Bone Cyst
The term ABC was first used by Jaffe and Lichtenstein to describe two examples of blood-filled cyts in which tissue from the cyst wall contained conspicuous spaces, areas of hemosiderin deposition, giant cells, and occasional bone trabeculae. In a subsequent publication, Jaffe chose the designation ABC as a descriptive term for this lesion to emphasize the blown-out appearance. Although the cause of this lesion is unknown, alterations in local hemodynamics related to venous obstruction or arteriovenous fistula are believed to play an important role. Some investigators believe that the lesion is caused by a trauma. Dahlin and McLeod postulated that it may be similar to and related to other reactive nonneoplastic processes, such as giant-cell reparative granuloma or traumatic reactions observed in periosteum and bone. ABC may arise de novo in bone, in which case no recognizable preexisting lesion can be demonstrated in the tissue, or it may be associated with various benign (e.g., giant-cell tumor, osteoblastoma, chondroblastoma, chondromyxoid
fibroma, fibrous dysplasia) and malignant (e.g., osteosarcoma, fibrosarcoma, or chondrosarcoma) lesions. The concept of ABC as a secondary phenomenon occurring in a preexisting lesion has been validated by several researchers. Some investigators, however, regard ABC as a reparative process, probably the result of trauma or tumor-induced anomalous vascular process.
fibroma, fibrous dysplasia) and malignant (e.g., osteosarcoma, fibrosarcoma, or chondrosarcoma) lesions. The concept of ABC as a secondary phenomenon occurring in a preexisting lesion has been validated by several researchers. Some investigators, however, regard ABC as a reparative process, probably the result of trauma or tumor-induced anomalous vascular process.
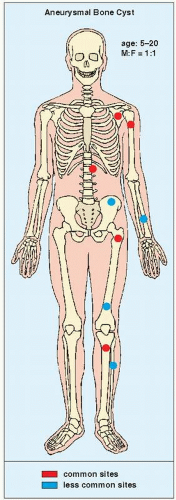
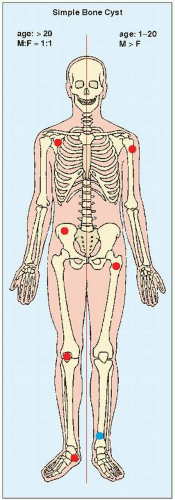 FIGURE 20.1 Skeletal sites of predilection, peak age range, and male-to-female ratio in SBC. The left half of the skeleton shows unusual sites of occurrence seen in an older patient population. |
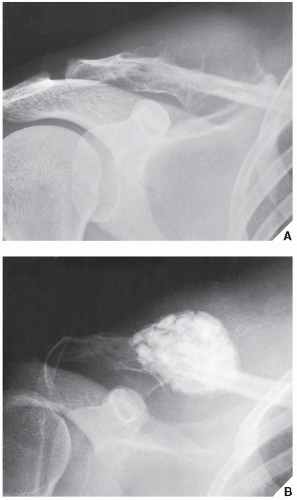
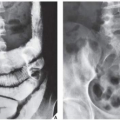
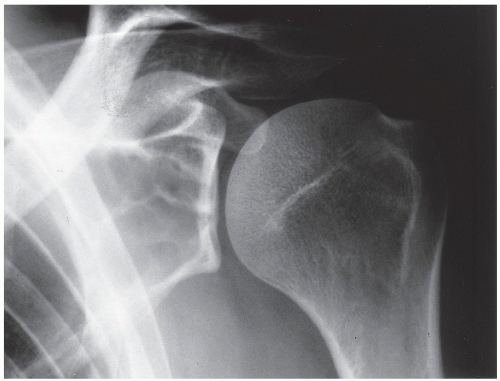 FIGURE 20.11 Intraosseous ganglion. An 18-year-old woman presented with left shoulder pain. Anteroposterior radiograph shows a radiolucent, trabeculated lesion in the glenoid, with the appearance of a SBC. Excisional biopsy was consistent with an intraosseous ganglion (see also Fig. 16.28A). |
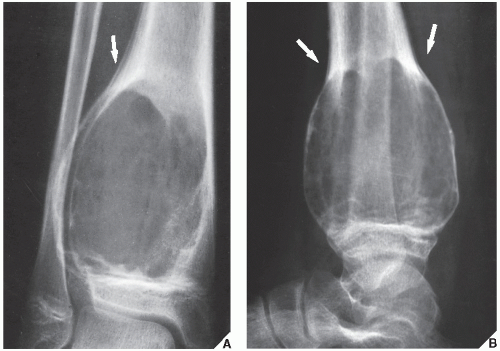
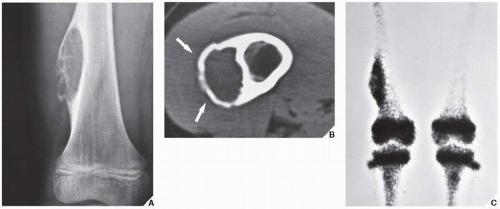
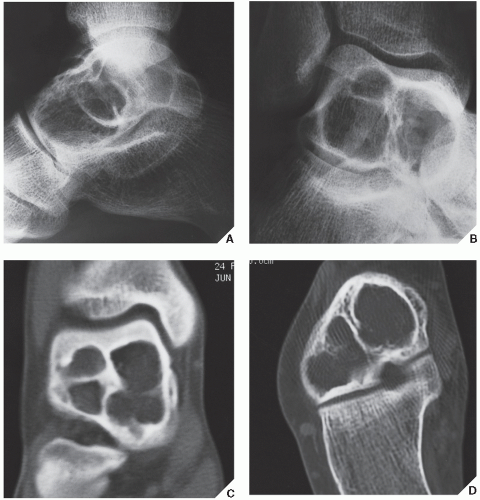
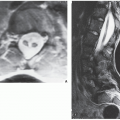
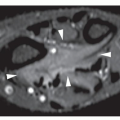
ABC constitutes approximately 6% of the primary lesions of bone and is seen predominantly in children; 90% of these lesions occur in patients younger than age 20 years. The metaphysis of long bones is a frequent site of predilection, although ABCs may sometimes be seen in the diaphysis of a long bone, as well as in flat bones such as the scapula or pelvis and even in the vertebrae (Fig. 20.12). As already stated, these lesions can develop de novo or as a result of cystic changes in a preexisting lesion such as a chondroblastoma, osteoblastoma, giant cell tumor (GCT), or fibrous dysplasia (Fig. 20.13). The radiographic hallmark of an ABC is multicystic eccentric expansion (blow-out) of the bone, with a buttress or thin shell of periosteal response (Figs. 20.14,20.15,20.16,20.17). Although conventional radiographs usually suffice for evaluating the lesion, computed tomography (CT), MRI, and radionuclide bone scan can be of further assistance. CT is particularly helpful in determining the integrity of the cortex (Fig. 20.17B, see also Fig. 20.19B). CT may also show internal ridges described on radiography as trabeculation or septation (Fig. 20.18). Fluid-fluid levels can also be demonstrated by this technique. These fluid levels are believed to represent the sedimentation of red blood cells and serum within the cystic cavities. To demonstrate this phenomenon, the patient must remain motionless for at least 10 minutes before scanning, and imaging must be performed in a plane perpendicular to the fluid levels.
MRI findings are rather characteristic and usually allow a specific diagnosis of ABC. These include a well-defined lesion, often with lobulated contours, cystic cavities with fluid-fluid levels, multiple internal septations, and an intact rim of low-intensity signal surrounding the lesion (Figs. 20.19,20.20,20.21,20.22,20.23). This rim has been described as an indicator of a benign process. The wide range of signal intensities within the cyst on T1- and T2-weighted sequences is probably caused by settling of degraded blood products and reflects intracystic hemorrhages of different ages.
Skeletal scintigraphy (see Fig. 20.17C) may occasionally be helpful because it reflects the vascular nature of the lesion. Some investigators have reported an increased uptake of radiopharmaceutical in a ring-like pattern around the periphery of ABC. Although this phenomenon is not specific for the lesion (it can also be observed in SBC and in bone infarct), the scintigraphic findings corroborate the radiographic presentation. Hudson, in his experience with 25 patients with ABC who underwent skeletal scintigraphy using technetium-99m methylene diphosphonate (99mTc-MDP) and 99mTc-pyrophosphate, found a correlation between the histopathologic features of the lesion, the amount and type of fluid contained within the cyst, and the scintigraphic pattern or intensity of uptake.
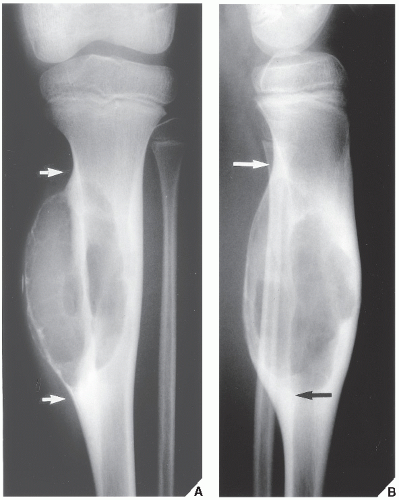
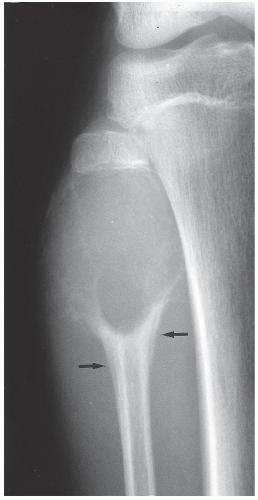 FIGURE 20.16 Aneurysmal bone cyst. A large, radiolucent expansive lesion in the proximal fibula of an 11-year-old girl reveals a buttress of periosteal reaction (arrows). |
Histologically, the ABC consists of multiple blood-filled sinusoid spaces alternating with more solid areas. The solid tissue is composed of fibrous elements containing numerous multinucleated giant cells and is richly vascular. The sinusoids have fibrous walls, often containing osteoid tissue or even mature bone. Focal or diffuse collections of hemosiderin or reactive foam cells may be seen in the fibrous septa.
Complications and Differential Diagnosis
The most common complication of an ABC is a pathologic fracture.
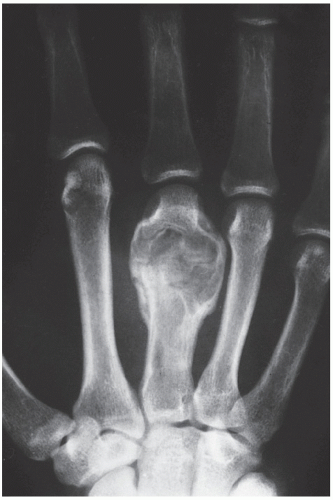
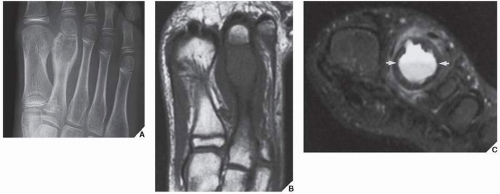
The conditions that should always be included in the differential diagnosis at any age are SBC, chondromyxoid fibroma, and GCT, which occurs after skeletal maturity when the lesion extends into the articular end of bone. The most critical points in differentiation of ABC from SBC are that the former is an eccentric, expansive lesion, invariably associated with some degree of periosteal reaction (usually a solid layer or solid buttress). The latter is a centrally located lesion, showing little if any expansion and exhibiting periosteal reaction only when a pathologic fracture has occurred. In thin bones, such as the ulna, fibula, metacarpals, or metatarsals, the characteristic eccentricity of ABC may be lost and, conversely, SBC may demonstrate expansive features (Fig. 20.24). Because the former contains solid tissue whereas SBC is a hollow structure filled with fluid, a fallen fragment sign (if present) is a good differential feature, pointing to the latter diagnosis. Chondromyxoid fibroma may be indistinguishable from ABC because both lesions are eccentric, expansive, and usually affect the metaphysis, exhibiting a reactive sclerotic rim and the aforementioned solid periosteal reaction (usually in the form of a buttress). CT and MRI are sometimes effective in making this distinction if they identify fluid-fluid levels, a phenomenon that points to the diagnosis of ABC because chondromyxoid fibroma is a solid lesion. In the mature skeleton, GCT may closely mimic ABC, although it usually is not associated with a periosteal reaction and rarely exhibits a zone of reactive sclerosis. Giant cell reparative granuloma (so-called solid aneurysmal bone cyst) may be indistinguishable from the conventional ABC. This lesion, however, unlike true ABC, usually involves the short tubular bones of the hands and feet. The cortex is thin but is characteristically intact. Extension into the surrounding soft tissues is distinctly uncommon, and the periosteal reaction is usually absent (see later). In thinner bones, such as the fibula, metacarpals, or metatarsals, ABC caused by expansive growth may destroy the cortex, mimicking an aggressive tumor such as telangiectatic osteosarcoma. Conversely, it is important to remember that at times a telangiectatic osteosarcoma may masquerade as an ABC. Histopathologic differentiation is critical in these situations.
Treatment
The treatment for ABC consists of surgical removal of the entire lesion. At times, bone grafting to repair the resulting defect may be necessary
(Fig. 20.25). Recently, percutaneous injections of Ethiblock, an alcoholic (ethanol) solution of corn protein which has thrombogenic and fibrogenic properties, have been advocated. Recurrence of the lesion, however, is frequent.
(Fig. 20.25). Recently, percutaneous injections of Ethiblock, an alcoholic (ethanol) solution of corn protein which has thrombogenic and fibrogenic properties, have been advocated. Recurrence of the lesion, however, is frequent.
Solid Variant of ABC
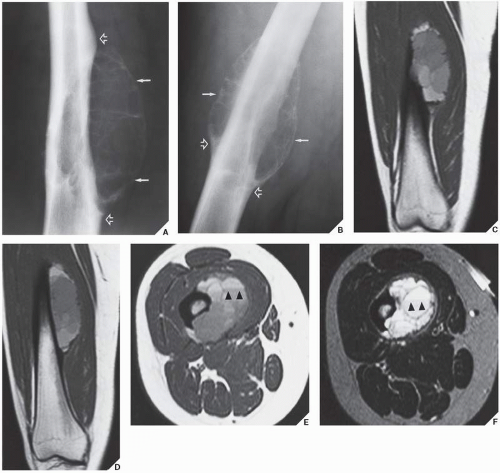
In 1983, Sanerkin and colleagues described a variant of ABC in which the predominant histology was that of the solid components of a conventional ABC. The histopathologic appearance of this lesion was very similar to that of another condition, reported originally by Jaffe in 1953 and later by Lorenzo and Dorfman in 1980, that represented a nonneoplastic hemorrhagic process in bones, termed giant cell reparative granuloma. The terms solid aneurysmal bone cyst and giant cell reparative granuloma are now being used interchangeably. These lesions are considered reactive and nonneoplastic, although they can lead to a mistaken diagnosis of malignancy. Although these lesions are seen primarily in craniofacial and short tubular bones of the hands and feet, they may also occur in the long bones, such as femur, tibia, and ulna. Radiography reveals that most of these lesions are expansive and eccentric in location, with variably aggressive features. At times, there is a thin shell of periosteal reaction indistinguishable from conventional ABC. MRI findings are variable, but most lesions show intermediate signal intensity on T1-weighted images, with heterogeneous but predominantly high signal intensity on T2 weighting (Fig. 20.26).
The areas of low signal on T2-weighted sequences represent mineralization within the lesion. Histopathologic examination of these lesions reveals fibrous stroma, an admixture of spindle cells, and many multinucleated giant cells. Occasional formation of osteoid and even mature bone trabeculae can be noted. Vascular spaces and hemorrhagic areas are also present. Some of these lesions have a histologic appearance similar to that of the so-called brown tumors of hyperparathyroidism. Treatment of these lesions usually consists of curettage. The recurrence rate, as recently reported from the Rizzoli Institute in Bologna, Italy, is close to 24%, whereas the Mayo Clinic reports approximately 39%.
The areas of low signal on T2-weighted sequences represent mineralization within the lesion. Histopathologic examination of these lesions reveals fibrous stroma, an admixture of spindle cells, and many multinucleated giant cells. Occasional formation of osteoid and even mature bone trabeculae can be noted. Vascular spaces and hemorrhagic areas are also present. Some of these lesions have a histologic appearance similar to that of the so-called brown tumors of hyperparathyroidism. Treatment of these lesions usually consists of curettage. The recurrence rate, as recently reported from the Rizzoli Institute in Bologna, Italy, is close to 24%, whereas the Mayo Clinic reports approximately 39%.
Giant Cell Tumor
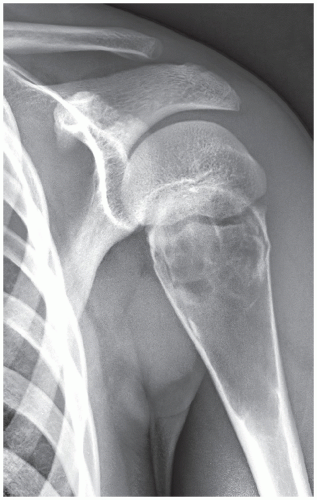
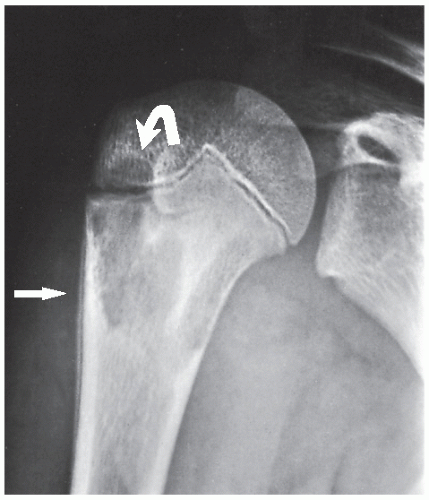
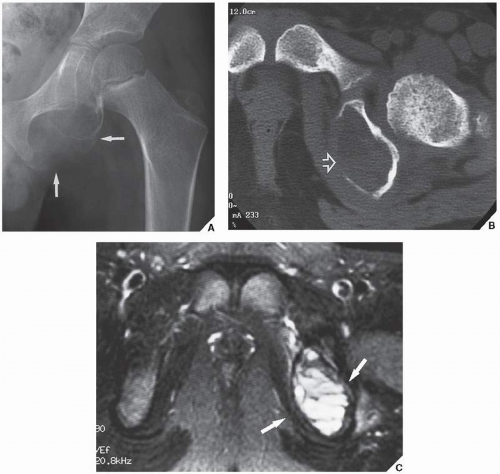
Also known as osteoclastoma, a GCT of bone is an aggressive lesion characterized by richly vascularized tissue containing proliferating mononuclear stromal cells and numerous uniformly distributed giant cells of osteoclast type. It represents approximately 5% to 8.6% of all primary bone tumors and approximately 23% of benign bone tumors; it is the sixth most common primary osseous neoplasm. Sixty percent of these lesions occur in long bones, and almost all are localized to the articular end of the bone. Preferred sites include the proximal tibia, distal femur, distal radius, and proximal humerus (Fig. 20.27). GCTs are seen almost exclusively after skeletal maturity, when the growth plate is obliterated. Most patients are between ages 20 and 40 years, and there is a female predominance of 2:1.
Multifocal GCTs are rare, accounting for less than 1% of all cases of GCT of bone. They occur most commonly in patients with Paget disease. Multiple lesions can be discovered synchronously or metachronously. The preferential locations are skull and facial bones in Paget disease, and small bones of the hands and feet in other patients.
Clinical symptoms in patients with solitary lesions are nonspecific. They include pain (usually reduced by rest), local swelling, and limitation of range of motion in the adjacent joint. When a lesion is located in the spine, neurologic symptoms may be present.
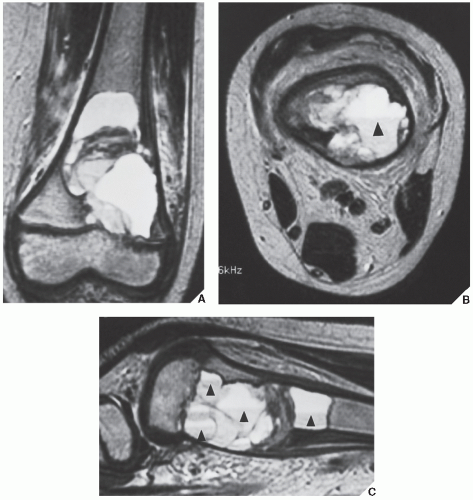
The imaging features of a GCT are characteristic. It is a purely osteolytic, radiolucent lesion with narrow zone of transition lacking sclerotic margins, revealing geographic bone destruction and usually no periosteal reaction (Figs. 20.28,20.29,20.30). Scintigraphy may show more intense uptake of the tracer around the periphery of the lesion than within the lesion itself, which Hudson calls a “donut configuration,” and is presumably caused by hyperemic changes in the bone surrounding the tumor. A soft-tissue mass may also be present, and CT or MRI is usually required for sufficient evaluation (Figs. 20.31,20.32,20.33). Approximately 5% of GCTs are malignant de novo. Having no characteristic imaging features, however, malignant lesions cannot be diagnosed radiologically (Figs. 20.34 and 20.35). It is also well known that benign GCT may evolve into a malignant lesion. Several authors have reported cases of malignant transformation of GCT of bone. In most cases, this transformation occurs after radiation therapy. Only a few cases have been reported of spontaneous malignant transformation after initial surgical therapy. Histologically, the secondary malignancies include malignant fibrous histiocytoma, fibrosarcoma, osteosarcoma, and undifferentiated sarcoma.
Histologically, a GCT is composed of a related dual population of mononuclear stromal cells and multinucleated giant cells. The tumor background contains varying amounts of collagen. Morphologically, the giant cells bear some resemblance to osteoclasts, and they display increased acid phosphatase activity.
Historically, the imaging appearance and staging of GCTs have not accurately reflected the ultimate clinical outcome, but nevertheless several investigators, including Enneking, Campanacci, and Bertoni, have developed staging systems based on imaging and histologic appearance of this tumor. The stage 1 lesion has an indolent radiographic and histologic appearance. The stage 2 lesion demonstrates a more aggressive radiographic appearance, with extensive remodeling of bone but intact periosteum and a benign histologic pattern. Stage 3 GCT reveals aggressive growth and extension into adjacent soft tissues but remains histologically benign, although distant metastases may occur.
Differential Diagnosis
Various lesions may be mistaken for GCT and, conversely, GCT can mimic other lesions that affect the articular end of a bone. Primary ABC rarely affects the articular end of a bone and occurs in a younger age group. However, after obliteration of the growth plate at skeletal maturity, this lesion may extend into the subarticular region of a long bone, becoming indistinguishable from a GCT. Occasionally, if the fluid-fluid level is demonstrated either on CT or on MRI examination, this feature is more consistent with ABC. However, it should be noted that ABC might sometimes coexist with other lesions, among them the GCT. The so-called solid ABC, or a giant cell reparative granuloma at the articular end, may have the same radiologic characteristics as a conventional GCT. Benign
fibrous histiocytoma, because of its frequent location at the end of a long bone, may appear identical to a GCT. Brown tumor of hyperparathyroidism is yet another lesion that can mimic GCT radiologically. However, the former lesion is usually accompanied by other skeletal manifestations of hyperparathyroidism, such as osteopenia, cortical or subperiosteal resorption, resorptive changes at the distal phalangeal tufts, or loss of the lamina dura of the teeth. Occasionally, an unusually large intraosseous ganglion may be mistaken for a GCT, although the former lesion invariably exhibits a sclerotic border. Some malignant lesions, such as chondrosarcoma, may extend into the articular end of bone and, particularly without radiographically identified calcifications, may closely mimic GCT. Myeloma and a lytic metastasis occupying subchondral segments of bone can usually be distinguished from GCT without much difficulty (the older age group in which the latter malignancies usually occur is a helpful hint), although at times the radiographic differences between the lesions may not be so obvious. Finally, on rare occasions, fibrosarcoma, malignant fibrous histiocytoma, or fibroblastic osteosarcoma (because of their purely lytic radiographic presentation) may exhibit some similarities to GCT.
fibrous histiocytoma, because of its frequent location at the end of a long bone, may appear identical to a GCT. Brown tumor of hyperparathyroidism is yet another lesion that can mimic GCT radiologically. However, the former lesion is usually accompanied by other skeletal manifestations of hyperparathyroidism, such as osteopenia, cortical or subperiosteal resorption, resorptive changes at the distal phalangeal tufts, or loss of the lamina dura of the teeth. Occasionally, an unusually large intraosseous ganglion may be mistaken for a GCT, although the former lesion invariably exhibits a sclerotic border. Some malignant lesions, such as chondrosarcoma, may extend into the articular end of bone and, particularly without radiographically identified calcifications, may closely mimic GCT. Myeloma and a lytic metastasis occupying subchondral segments of bone can usually be distinguished from GCT without much difficulty (the older age group in which the latter malignancies usually occur is a helpful hint), although at times the radiographic differences between the lesions may not be so obvious. Finally, on rare occasions, fibrosarcoma, malignant fibrous histiocytoma, or fibroblastic osteosarcoma (because of their purely lytic radiographic presentation) may exhibit some similarities to GCT.
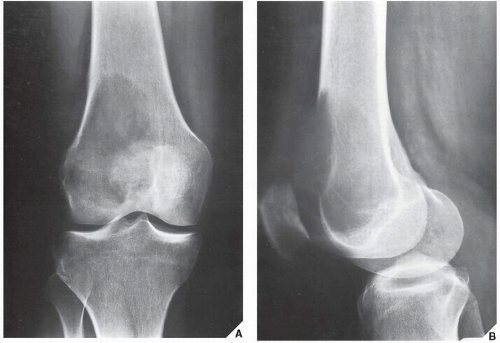
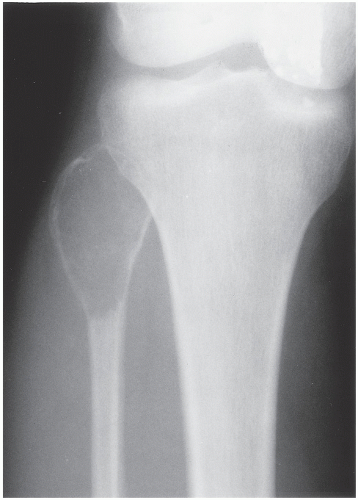 FIGURE 20.29 Giant cell tumor. Anteroposterior radiograph of the right knee in a 28-year-old woman shows an expansive radiolucent lesion in the head of the fibula. |
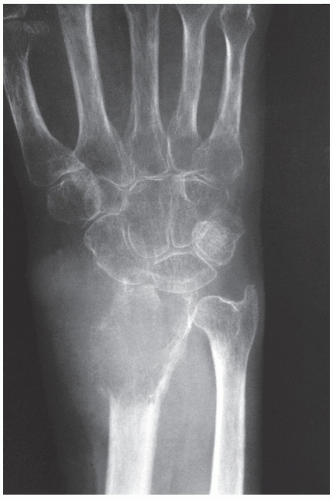 FIGURE 20.34 Giant cell tumor. Dorsovolar radiograph of the left wrist of a 56-year-old woman shows a lytic lesion of the distal radius that has destroyed the cortex and that extends into the soft tissues. Despite this aggressive radiographic presentation, on histopathologic examination the tumor had a typically benign appearance, without malignant features. After wide resection, a 5-year follow-up showed no evidence of recurrence or of distant metastases.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|