Fig. 21.1
(a) Segmental anatomy of the liver from Couinaud. (b) Common anatomic variants. Segments IV, V, VI, VIII
The right hepatic lobe is divided into anteromedial (segments V, VIII) and posterolateral sectors (segments VI, VII) by the right hepatic vein. The right hepatic duct drains segments V, VI, VII, and VIII. The right portal vein separates the superior segments (VII and VIII) from the inferior segments (V and VI). The right posterior and anterior ducts merge close to the hilum. The right hepatic duct is shorter than the left hepatic duct. Segment I had a variable biliary drainage. Most of the time segment I drains bilaterally (80 %), in the left hepatic duct (15 %) and in the right hepatic duct (5 %).
Numerous anatomic variants are described. Most of them involve the right segments V, VI, and VIII. No anatomic variant of segment VII has been reported. On the left side, anatomic variants of segment IV have been found (Fig. 21.1b) [1].
Transhepatic Cholangiography
Percutaneous transhepatic cholangiography (PTC) is the first step for all percutaneous biliary interventions. When MR is inconclusive, cholangiography is useful to differentiate between types of neonatal cholestases treatable with surgical or noninvasive approaches.
The initial puncture is performed with a 22-gauge Chiba needle. In case of biliary dilatation, the puncture is performed under ultrasonographic guidance. If the bile ducts are not dilated, the standard approach (described below) is recommended.
The puncture site is selected below the lateral costophrenic angle on full inspiration. The needle is inserted in the midaxillary line along the superior aspect of the lower rib to avoid the neurovascular intercostal bundle. The needle is advanced parallel to the tabletop in a 20–30° cranial direction toward a point lateral to the thoracic spine.
Classically, during the withdrawal of the needle, diluted contrast medium is gently injected until opacification of the bile ducts is seen. To minimize administration of parenchymal contrast material that will obscure the field of view, bile can be aspirated prior to injection. As an alternative technique, saline is injected while monitoring for intraluminal flow using ultrasound in order to avoid diffusion of contrast in the parenchyma and subsequent decreased visualization on fluoroscopy.
If the biliary system is not adequately opacified through the Chiba needle, a 0.018-in. mandril or hydrophilic wire can be used to insert a 3 Fr dilator through which contrast can then be injected.
The technical success of the procedure is almost 100 % if the biliary tree is dilated, but drops to 50 % when the ducts are not dilated. In pediatrics, when the biliary tree is not dilated, we frequently puncture the gallbladder for the biliary opacification. Biliary drainage is described below.
Complications
The complications of biliary access are rare (<5 %) and include sepsis, bleeding, biliary leakage, and pneumothorax [2].
Cholangiographic Diagnosis of Specific Biliary Diseases in Children
Biliary Atresia
Biliary atresia is the most common cause of neonatal cholestasis and is the main indication for liver transplantation in children. The incidence is 1/9,600 in Japan and 1/14,000 in the USA [3] with a female preponderance. The cause of the disease remains unknown [4–6]. Clinical presentation includes the presence of a hard liver and white stools. A nonsyndromic form is associated with 90 % of biliary atresia cases. In 10 %, biliary atresia can be associated with polysplenia, cardiac and pulmonary malformations, situs inversus, preduodenal portal vein, interrupted inferior vena cava, and intestinal malrotation [7]. Those abnormalities should be systematically searched for in the evaluation of biliary atresia patients.
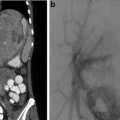
Three types of biliary atresia are described. In type 1 (5 %), the obstruction occurs at the level of the common bile duct with the presence of a gallbladder. In type 2 (3 %), the obstruction occurs more proximally within the common hepatic duct. The small gallbladder contains no bile. In type 3 (90 %), the level of obstruction is at the porta hepatis. Examples of biliary atresia are shown in Figs. 21.2 and 21.3.
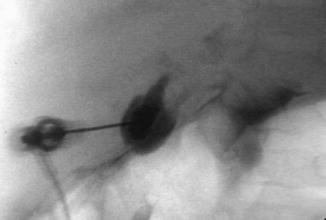
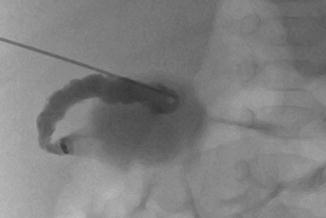

Fig. 21.2
Biliary atresia. Percutaneous transhepatic cholangiography. Puncture and opacification of the micro gallbladder demonstrate the absence of normal bile duct

Fig. 21.3
Puncture of the gallbladder. The opacification demonstrates an abnormal gallbladder connected to a large cyst but without communication with the duodenum, indicating the biliary atresia
Differential diagnosis includes rare surgical causes (congenital obstructive choledochal malformations [8] and bile plug syndrome) and medical causes of neonatal cholestasis (neonatal hepatitis, α1-antitrypsin deficiency, cystic fibrosis). The diagnosis of biliary atresia is mandatory in order to delay or prevent biliary cirrhosis and the subsequent need for a liver transplantation. Accordingly, a Kasai procedure (hepatoportoenterostomy) should be performed within 40–60 days after birth [6, 8]. The majority of cases will eventually require liver transplantation.
Choledochal Cyst
Choledochal cyst represents a rare cause of neonatal cholestatic jaundice. Development of a choledochal cyst may result from an abnormally long pancreaticobiliary duct allowing reflux of pancreatic enzymes into the biliary ducts [9–12]. Female preponderance occurs in a ratio of 4:1. Clinical manifestations such as abdominal pain, jaundice, or mass are nonspecific. Diagnosis is usually made before 10 years of age; some cases are discovered in utero and in adulthood [9, 10]. Differential diagnoses include biliary lithiasis, pancreatic pseudocyst, hepatic cyst, primary sclerosing cholangitis, enteric duplication, biliary hamartomas, microabscess, and biliary papillomatosis.
Complications include lithiasis leading to infection and even cystic rupture, biliary cirrhosis, and cholangiocarcinoma [10].
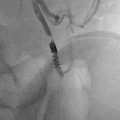
Five types of congenital biliary cysts have been described [13]. Type 1 is the most frequent (80–90 % of cases) in which the cystic malformation is confined to the extrahepatic ducts (Fig. 21.4). Three subtypes are described depending on the extension of the disease. In subtype 1a, the “diffuse type,” all extrahepatic ducts are involved, and the gallbladder drains in the cyst. In subtype 1b, called “focal type,” only a focal segment of the extrahepatic duct is involved, whereas subtype 1c is characterized by a fusiform enlargement of the choledochal duct, while the common hepatic duct is cylindrical. In type 2 (2 % of cases), the choledochal cyst is a diverticulum of the extrahepatic duct. In type 3 (1.4–5 % of cases), a choledochocele is located in the intraduodenal segment of the choledochal duct. In type 4, multiple cystic dilatations are encountered along both intra- and extrahepatic ducts (type 4a) (Fig. 21.5) or limited to the extrahepatic ducts (type 4b). Type 5 is known as Caroli’s disease. This Mendelian recessive disease (same PKHD1 gene as in the recessive polycystic kidney disease) is characterized by a nonobstructive cystic dilatation of the intrahepatic ducts that can be focal or diffuse.
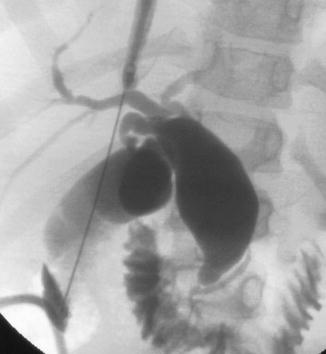
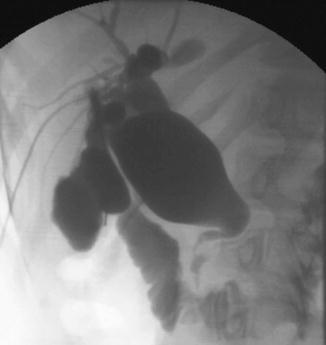

Fig. 21.4
Cholangiogram demonstrates a type 1a choledochal cyst

Fig. 21.5
Puncture of the gallbladder with a Chiba needle. The opacification reveals an extrahepatic and intrahepatic cystic dilatation diagnostic of type 4a choledochal cyst
Biliary atresia of the distal common bile duct can be associated with a choledochal cyst. When the dilated common bile duct does not communicate with the duodenum, the diagnosis is biliary atresia of the distal choledochus [8–10].
Chronic biliary stasis may lead to the same complications as listed above. Although there is no primary involvement of the extrahepatic tract, the latter can be secondarily modified in the course of recurrent infectious cholangitis.
MR cholangiography has predominantly replaced percutaneous cholangiography for diagnosis of choledochal cysts. It is useful to describe the cystic anomalies and define their type. In Caroli’s disease, the “central dot sign,” considered as highly suggestive, results from portal vein enhancement that is surrounded by cystic intrahepatic biliary ducts [14]. The pancreaticobiliary junction can also be analyzed by MR cholangiography.
Percutaneous or endoscopic retrograde cholangiography can be useful to confirm the diagnosis, determine the type of disease, and precisely analyze the pancreaticobiliary channel and its length.
Paucity of Intrahepatic Bile Ducts
Definitive diagnosis of this disease is histopathological requiring a liver biopsy specimen of adequate size (large) to demonstrate the paucity of bile ducts (Fig. 21.6) [15]. This is a common cause of neonatal or pediatric cholestatic jaundice. Two forms are described based on the presence or absence of other malformations. The nonsyndromic form can be idiopathic or associated with metabolic or viral diseases, chromosomal alterations, neoplasia, cystic fibrosis, and altered bile acid metabolism. The syndromic form is known as Alagille syndrome [16], an autosomal dominant inheritance disorder with variable expressivity that associates at least three of the five following major features: chronic cholestasis, characteristic facies, peripheral pulmonary artery stenosis, butterfly vertebrae (anterior rachischisis), and posterior embryotoxon. Prognosis varies, especially with the cardiovascular abnormalities. Pediatric liver transplantation is necessary in approximately 40 % of cases [17, 18].
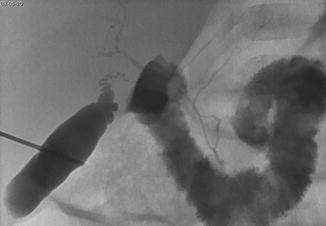

Fig. 21.6
Patient with Byler disease. Percutaneous transhepatic cholangiography demonstrates ductular hypoplasia
Infantile Sclerosing Cholangitis
Sclerosing cholangitis in children is a rare and heterogeneous condition, resulting from an inflammatory obliterative fibrosis that affects intra- and extrahepatic biliary ducts. The disease causes hepatic insufficiency and cirrhosis and carries a risk of malignant transformation into cholangiocarcinoma. Cholestatic jaundice is the main clinical finding that may occur in the early neonatal period but can be seen much later in the infant’s life [19].
Etiologies vary and may remain unknown [20]. Immunodeficiency, inflammatory bowel disease, cystic fibrosis, and histiocytosis are classical etiologies of sclerosing cholangitis. Despite treatment of the underlying disease, liver transplantation is often needed.
MR cholangiography shows irregular biliary dilatation and beading which are segmental and involve multiple focal hepatic territories. The intrahepatic biliary ducts are always abnormal. The extrahepatic ducts are abnormal in 60 % of cases.
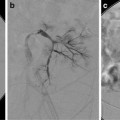
Percutaneous or endoscopic retrograde cholangiography shows intrahepatic biliary duct involvement in 100 % of cases and extrahepatic involvement in 60 % of cases [21]. There is diffuse stenosis of the bile ducts with focal dilatations between strictures providing the characteristic beaded appearance (Fig. 21.7). Different segmental hepatic areas are involved [21, 22]. Liver biopsy is useful when cholangiography is normal [23].
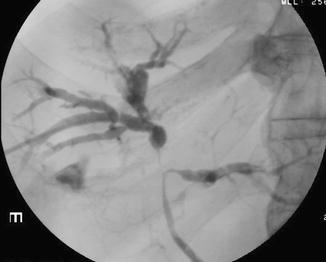

Fig. 21.7
Cholangiography in infantile sclerosing cholangitis shows irregular biliary dilatation, beading, and stenoses
Percutaneous Transhepatic Biliary Drainage
In pediatrics, percutaneous transhepatic biliary drainage is indicated in the treatment of bile plug syndrome, choledocholithiasis, tumor, and strictures associated with liver transplant. The most common obstructive tumors seen in the pediatric age group are rhabdomyosarcoma and inflammatory pseudotumor. Other causes of biliary strictures include postoperative injury after cholecystectomy, hepatic resection, laparoscopy, trauma, acute or chronic pancreatitis, primary sclerosing cholangitis, or radiation.
After opacification of the biliary tree with PTC, drain insertion is performed. When PTC is performed through a central duct, a second puncture is often needed to insert the drain into a more appropriate peripheral segmental duct. A coaxial dilator system (such as a Neff or Accustick Set) is used to traverse the hepatic parenchyma, provide stable access for biliary tract intervention, and allow upsizing of the 0.018″ mandril or hydrophilic wire to a 0.035″ system. The three components are an outer sheath, an inner tapered introducer, and an innermost metal stiffening cannula. Placement of a secondary safety wire can be considered for additional stability.
After introducing a guidewire into the biliary tract, the next step is to cross the stricture and gain access to the bowel for internal or internal–external biliary drainage. This is generally achieved with an angiographic catheter and hydrophilic guidewire. After crossing the stricture with a guidewire, balloon dilatation is performed (2–6 mm in diameter). The stricture is dilated serially until it can accommodate an appropriately sized drainage catheter (6–14 Fr). The balloon is inflated for approximately 1 min per inflation. Dilatation must usually be performed progressively to avoid laceration and massive bleeding into the biliary system. Dilatation may be repeated several times to obtain a good duct caliber. Severe fibrotic strictures can be managed with high pressure balloons. Once the inflation is initiated, a waist is seen at the stricture site that usually disappears with increasing pressure as the stenosis subsides. The hydrophilic guidewire is removed, and a stiff 0.035″ or 0.038″ Teflon-coated metallic wire is placed, and the final drainage catheter is advanced over the guidewire (Figs. 21.8 and 21.9). Standard or handmade transanastomotic internal–external biliary drainage catheters ranging from 6 to 14 Fr in size (Boston Scientific, Natick, MA) are used. Occasionally, additional side holes must be made in the catheter if the holes provided by the manufacturers do not allow adequate drainage. It is paramount to adjust the size and distribution of the holes to fit with the patient’s anatomy. In other words, one must assess the exact location of the side holes to optimize the drainage without extrahepatic biliary leak. In order to do so, a guidewire is placed within a transhepatic catheter advanced in the third portion of duodenum. The ideal location of the most proximal and distal side holes is marked by bending the guidewire prior to its removal. Any necessary additional side holes are then created on the drainage catheter based on these measurements. After the placement of the catheter, contrast injection is recommended to ensure proper opacification of the biliary tree, nonopacification of the portal or hepatic venous system, and absent peritoneal extravasation.
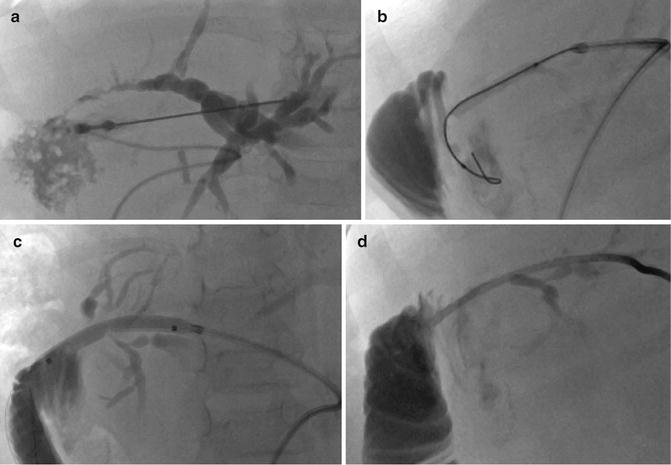
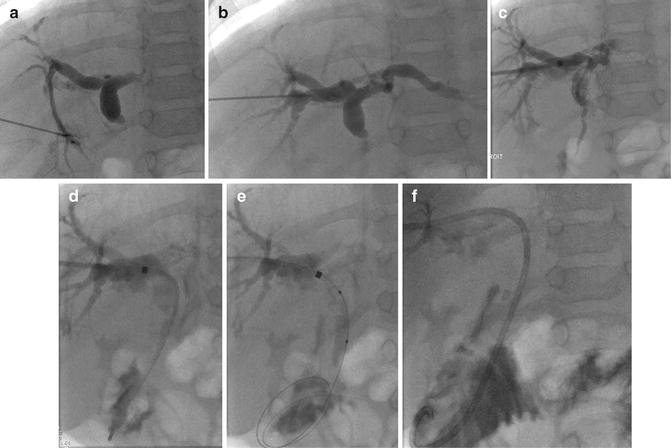

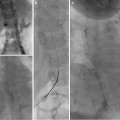
Fig. 21.8
(a) Puncture of the biliary branch of segment II showing a stenosis of the transplant’s biliary–bowel intestinal anastomosis. (b) A guidewire is inserted followed by the placement of the introducer (Neff set). The end of the wire is in the blind end of the Roux-en-Y loop. The guidewire must be in the jejunal loop. (c) A progressive dilatation from 4 to 6 mm balloon size was performed. (d) An internal–external biliary catheter was placed

Fig. 21.9
(a) Image from the initial percutaneous transhepatic cholangiogram demonstrating injection into a biliary radicle with filling of the biliary tree. A severe stenosis was demonstrated without opacification of the bowel. (b) A distal branch of the segment VII was punctured under ultrasound. (c) The introducer sheath was installed. (d) A Terumo guidewire with a glide catheter (4 Fr) was used to cross the stenosis. (e) Progressive dilatation of the choledochal anastomosis was done with 4–7 mm balloons. (f) An internal-external biliary catheter was installed
Sometimes a two-stage procedure is necessary. Patients with obstructed, infected biliary systems can become acutely septic as a result of bile duct manipulation. Some strictures are difficult to cross due to regional anatomy or edema of the area. In these instances, a drain is left in the biliary tree proximal to the stenosis. Secondary placement of an internal–external drain can be undertaken after a minimum of 48 h of external drainage and antibiotic treatment when the patient has defervesced.
Percutaneous cholangiogram is an effective method to identify the presence of biliary strictures. The success rate of PTC is 96 %, and the reported success rate of percutaneous biliary drainage is 89 % in pediatric liver transplant recipients [24, 25]. Sunku et al. reported a 100 % technical success rate for PTC and balloon dilatation. Stricture recurrence is a real drawback occurring in 66 % of cases in Sunku et al. series. The recurrence rate was 45 % for anastomotic strictures, 90 % for intrahepatic strictures, and 100 % for the cases with both an anastomotic and intrahepatic strictures [24].