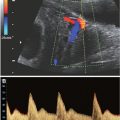
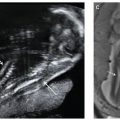
FIGURE 9.1: Intracranial ultrasound findings in fetuses with open NTDs: banana sign (A), lemon sign (B).
The majority of studies evaluating the accuracy of ultrasound have been performed in high-risk populations (i.e., patients with an elevated maternal serum AFP). More recent studies have suggested that routine second trimester ultrasonography actually may be more likely to identify an open NTD compared with routine maternal serum AFP screening in the general population.21 Finally, first-trimester screening for open NTDs is currently under active investigation. It has been proposed that in fetuses with open spina bifida, the downward displacement of the fetal brain will lead to nonvisualization of the fourth ventricle in early gestation. The fourth ventricle, described as the “intracranial translucency,” is typically visualized in the midsagittal view of the fetal face used to obtain first-trimester nuchal translucency measurement in normal fetuses. While larger prospective studies are warranted, preliminary results demonstrate promise.22,23 In addition, recent studies also have demonstrated that a small biparietal diameter in the first trimester of pregnancy may be a subtle marker for open spina bifida.24,25 In a retrospective review, Bernard and colleagues24 observed a 10-fold increased risk of open spina bifida when the biparietal diameter measured less than the 5th percentile between 11 and 14 weeks. Combining this potential for a first-trimester screening modality with the high detection rates observed in second-trimester ultrasonography, the future role of maternal serum AFP screening for open NTDs is uncertain.
FACTORS AFFECTING MATERNAL SERUM AFP INTERPRETATION
Accurate pregnancy dating is one of the key components to successful maternal AFP serum screening. Maternal serum AFP concentrations increase by 15% to 20% per week between 16 and 22 weeks; therefore, inaccurate dating may lead to erroneous results. The optimal timing to perform maternal serum AFP screening is between 16 and 18 weeks. Prior to this gestational age, there is considerable overlap between affected and unaffected pregnancies.3 First-trimester crown-rump length measurement is the preferred method for accurate pregnancy dating; however, fetal biometry may be used after 14 weeks’ gestation. Given that biparietal diameter measurements may lag in fetuses with open NTDs, attention to other biometric parameters is necessary.
Other factors that must be accounted for when calculating maternal serum AFP concentration include maternal ethnicity, weight, and the diagnosis of diabetes mellitus. African Americans have a 10% to 15% higher serum AFP level compared with other races, despite the fact that African Americans have a lower incidence of NTDs overall.26,27 While there may be small differences in serum AFP levels between other racial groups, these differences are not large enough to necessitate adjustment. Given that increasing maternal weight reflects an increased volume of distribution, higher body weights will result in a relative dilution of maternal serum AFP levels. Prior studies have shown that adjustment for maternal weight actually increases the detection rate of open NTDs and decreases the FPR.28 The relationship between maternal serum AFP levels and diabetes mellitus remains controversial. It has been shown that serum AFP levels are approximately 20% lower in diabetic patients compared with nondiabetic patients; however, it remains uncertain whether the cause for this is the disease state itself or the state of glycemic control.29 Notably, Baumgarten and Robinson30 demonstrated a significant inverse relationship between maternal serum AFP and glycosylated hemoglobin, supporting the theory that uncontrolled glucose levels are the driving force behind this observed decrease. Additionally, it has been proposed that adjustment for maternal weight alone may be adequate. Although most laboratories continue to correct for diabetes in their AFP MoM calculation, the most recent literature suggests that this is likely unnecessary, regardless of insulin requirement.31,32 Finally, maternal serum and amniotic fluid AFP levels are ineffective following multifetal or selective reduction procedures.33 Ultrasound remains the optimal choice for open NTD screening in these patients.
ULTRASOUND EVALUATION FOR ELEVATED AFP LEVELS
False Positive Results
The first step in evaluating an elevated maternal serum AFP level is to perform an ultrasound to assess gestational age, if this has not previously been done. In approximately 50% of cases, underestimation of gestational age is identified, resulting in a false positive value.3 In such cases, the initial serum AFP value can be adjusted, and often no further testing is needed. Fetal viability also should be assessed, as fetal demise can lead to an elevated maternal serum AFP level secondary to disruption in the maternal–fetal interface. Finally, it is imperative to evaluate for the presence of a multifetal gestation. Increased placental mass in multifetal gestations leads to increased AFP, approximately twofold higher in twin gestations compared with singletons. Typically, ultrasound evaluation for open NTDs is thought to be the gold standard evaluation for multifetal pregnancies; however, some laboratories do report an adjusted serum AFP value for twin gestations. Using this approach, either the maternal serum AFP MoM is divided by the median AFP level in unaffected twin pregnancies or a higher singleton threshold is used to define a positive screen. The issue with this approach is that a single serum value is being used to provide information on multiple fetuses. Cuckle and colleagues34 demonstrated that by using a 2.5-MoM threshold, such as in a singleton pregnancy, the detection rate for open spina bifida in twin gestations was 89%; however, the FPR was as high as 30%. In order to maintain a FPR similar to that of singleton gestations, a 5.0-MoM threshold would need to be used, resulting in an open NTD detection rate of only 39% in twin pregnancies.
Maternal–fetal hemorrhage can also result in a falsely positive elevated maternal serum AFP level. While this can often be elicited through a maternal history of vaginal bleeding, not all cases of maternal–fetal hemorrhage will lead to recognizable clinical signs. Ultrasound evaluation may demonstrate subchorionic hematoma, retroplacental hematoma, or placental sonolucencies in such cases.35,36 Placental implantation site abnormalities such as placenta accreta have also been reported to be associated with elevated maternal serum AFP levels; therefore, ultrasound of the placental site is warranted, especially in cases of placenta previa and a prior uterine scar.37,38 Other placental findings associated with elevated maternal serum AFP levels include chorioangiomas, angiomyxomas, and placental hypertrophy.39,40 Rarely diagnosed during pregnancy, ovarian germ cell tumors and hepatic tumors also can cause elevated serum AFP levels; however, the magnitude of AFP increase is typically much higher than is typically observed in obstetric screening programs.
Other Fetal Anomalies
Apart from open NTDs, elevated maternal serum AFP levels also have been associated with other fetal malformations. The second most common group of anomalies associated with elevated AFP levels are fetal abdominal wall defects, including omphalocele, gastroschisis, and bladder extrophy. As in the case of NTDs, these open defects allow for leakage of fetal serum into the amniotic fluid, resulting in high amniotic fluid and maternal serum AFP concentrations. Saller and colleagues41 observed that pregnancies with gastroschisis and omphalocele had elevated AFP levels compared with the unaffected population, 9.42 and 4.18 MoMs, respectively. Other studies have demonstrated a greater screening sensitivity for gastroschisis compared with omphalocele at any given AFP threshold.42
Congenital skin disorders such as epidermolysis bullosa and aplasia cutis have also been associated with elevated AFP levels. This is hypothesized to be caused by increased diffusion of fetal AFP through the weeping skin lesions and into the amniotic fluid. Reflux of intestinal contents in cases of duodenal atresia, annular pancreas, and intestinal atresia as well as reflux of lung fluid in cases of congenital lung lesions can also cause AFP elevations. A full listing of fetal anomalies reported to be associated with elevated AFP levels is shown in Table 9.1.1,3
Fetal Anomalies Associated with Maternal Serum AFP Elevations
Open NTDs Gastroschisis Omphalocele Bladder extrophy Esophageal atresia Duodenal atresia Annular pancreas Pilonidal cysts Autosomal recessive polycystic kidney disease Epidermolysis bullosa Aplasia cutis Bilateral renal agenesis Sacrococcygeal hematoma Congenital cystic adenomatoid malformation Cystic hygroma Acardiac twin Obstructive uropathy Osteogenesis imperfecta Triploidy |
AFP, alpha-fetoprotein.
Although extremely rare in the general population, congenital nephrosis should be considered to be a potential diagnosis in cases of an extremely elevated AFP level and normal ultrasound findings.43 This autosomal recessive disorder is most common in Finland, where the incidence ranges from 1 in 2,600 to 1 in 8,000 pregnancies.44 This lethal disorder causes early renal failure, and death typically occurs in infancy or early childhood. Abnormal filtering capacity of the fetal glomeruli is thought to cause extreme fetal proteinuria. Consequently, elevated amniotic fluid AFP levels are present. Maternal serum levels are usually elevated on the order of 5 to 6 MoMs, whereas amniotic fluid AFP levels are typically elevated to >10.0 MoMs.45 While the fetal kidneys may appear slightly enlarged and echogenic, ultrasound is typically normal in these fetuses. Placentomegaly may occur; however, this finding usually is not observed until the third trimester. Definitive diagnosis is made by electron microscopy of a renal biopsy. In utero kidney biopsy has been suggested and successfully performed in order to make an antenatal diagnosis in cases with high suspicion for this disorder.46
Unexplained Elevated AFP
In approximately 1% of patients with an elevated AFP level, no apparent structural cause can be identified. However, these patients still remain at increased risk for adverse pregnancy outcomes, including fetal loss, preterm delivery, fetal growth restriction, oligohydramnios, placental abruption, and intrauterine fetal death.47–49 Milunsky and colleagues47 demonstrated that patients with an elevated maternal serum AFP level were at a twofold increased risk for preeclampsia, a threefold increased risk for placental abruption, a fourfold increased risk for low birth weight, and an eightfold increased risk for fetal death. Subsequently, Waller and colleagues conducted a case-control study and again demonstrated that elevated maternal serum AFP levels were associated with intrauterine fetal demise. The odds ratio for fetal death in that study was 10.4 (95% CI, 4.9 to 22.0) in the setting of AFP levels exceeding 3.0 MoMs.50 Most interestingly, that study also showed that the risk of intrauterine fetal death persisted through the third trimester, suggesting that there is potential for these high-risk patients to be identified and offered increased antenatal surveillance. Most recently, elevated maternal serum AFP levels have been linked to an increased risk of sudden infant death syndrome (SIDS); however, this association may be biased by an increased incidence of fetal growth restriction and preterm delivery in those patients.51 Despite these associations, the majority of women with an unexplained elevated AFP level do have a normal pregnancy outcome.
The optimal management strategy for patients with an unexplained elevated AFP level remains unclear. A suggested algorithm for the evaluation and management of these patients is shown in Figure 9.2. It is hypothesized that these unexplained elevations may be due to abnormal placentation; therefore, it might be possible to predict which patients are at highest risk for adverse outcome by indirectly evaluating the placental circulation through uterine artery Doppler studies. Initial studies evaluating the utility of uterine artery Doppler studies in predicting preeclampsia and fetal growth restriction produced a wide range of sensitivities and predictive values, with significant variation depending on the parameter studied (i.e., pulsatility index [PI], resistance index [RI], or diastolic notching).52–54 A recent systematic review and meta-analysis demonstrated that an increased uterine artery PI with notching was the best predictor of preeclampsia with a positive likelihood ratio (LR) of 21.0 in high-risk populations and 7.5 in low-risk populations.55

FIGURE 9.2: Suggested algorithm for the evaluation and management of elevated maternal serum AFP.
Relatively few studies specifically have addressed the role of uterine artery Doppler studies in populations with unexplained elevated maternal serum AFP levels. Aristidou and colleagues56 reported that the presence of diastolic notching was a good predictor of poor perinatal outcome in patients with an elevated maternal serum AFP level. Bromley and colleagues57 demonstrated similar results; however, they observed that only severe grade II notching was associated with a significant increase in adverse outcomes (RR 3.4, 95% CI, 1.9 to 6.0). Finally, Konchak and colleagues demonstrated that both uterine artery notching and a RI > 95th percentile were predictive of adverse outcomes in this population. The best test characteristics were observed for the prediction of preeclampsia. Uterine artery notching demonstrated an 83.3% sensitivity and 95.6% specificity for preeclampsia, whereas an elevated RI demonstrated an 83.3% sensitivity and a 93.8% specificity for preeclampsia. Lower sensitivities were noted for the outcomes of fetal growth restriction and preterm delivery. Despite these results, PPVs ranged only between 44% and 55%.58 While second-trimester uterine artery Doppler studies can be used in the evaluation of patients with an unexplained elevated maternal serum AFP level, there remain no specific guidelines for management and surveillance in these patients.
Umbilical artery Doppler studies as well as ultrasound evaluation of placental morphology have also been proposed as tools to evaluate patients with an unexplained elevated AFP level. Abnormal umbilical artery Doppler studies alone have not shown to be predictive of adverse outcomes in this population; however, in fetuses with identified fetal growth restriction, umbilical artery Doppler interrogation is useful in evaluating for worsening placental dysfunction.57 Abnormal placental thickness, texture, and shape on ultrasound, especially in the setting of abnormal uterine artery Doppler studies may also be a predictor of adverse perinatal outcome in patients with elevated AFP levels.59
Given the increased risk for fetal growth restriction in these patients, serial ultrasound assessments for fetal growth are warranted in the third trimester of pregnancy. If abnormal growth is detected, then umbilical artery studies and antenatal testing with biophysical profiles or nonstress tests should follow. It is unclear whether the risk for intrauterine fetal demise in these patients is independent of fetal growth restriction; therefore, many institutions propose antenatal testing even in the absence of fetal growth abnormalities. In a 2001 retrospective cohort study of 136 patients with unexplained elevated maternal serum AFP levels, Huerta-Enochian and colleagues49 demonstrated that intensive antenatal monitoring consisting of twice weekly nonstress tests and amniotic fluid volume assessment did not achieve earlier or improved detection of adverse outcome compared with routine prenatal care. The decision to perform antenatal testing in these patients remains an individualized decision between provider and patient.
USE OF BIOCHEMICAL MARKERS IN THE FIRST TRIMESTER
Aneuploidy Screening
In the mid-1990s, it was discovered that first-trimester alterations in pregnancy-associated plasma protein-A (PAPP-A) and free β-human chorionic gonadotropin (β-hCG) were associated with an increased risk for fetal aneuploidy.60–62 These biochemical markers are placentally-derived, pregnancy-specific glycoproteins which are detectable in maternal serum. In pregnancies affected by trisomy 21, PAPP-A is reduced to 0.4 MoMs, and free β-hCG is increased to 1.98 MoMs between 9 and 11 weeks’ gestation, on average. When combined with maternal age, low PAPP-A alone will identify approximately 50% of pregnancies affected by trisomy 21, whereas elevated free β-hCG alone will detect only 40% of pregnancies affected by trisomy 21.63 When combining both PAPP-A and free β-hCG with maternal age, that detection rate increases from 60% to 65% at an FPR of 5%.62
Prior studies have evaluated the impact of measuring the intact hCG molecule rather than the free β-hCG subunit, as discussed above. Although both serum analytes are increased in fetuses affected by Down syndrome, levels of intact hCG do not begin to increase in affected fetuses until approximately 11 weeks compared with levels of free β-hCG, which begin to increase at 9 weeks. In a meta-analysis by Evans and colleagues,64 the use of the free β-hCG subunit achieved a higher detection rate of trisomy 21 with a lower FPR compared with the intact hCG molecule. Today, most first-trimester aneuploidy screening protocols use the free β-hCG subunit; however, its impact on screening efficiency is likely greater during the earlier gestational age window of first-trimester serum screening.
In addition to these serum analytes, the discovery that an increased size of the fluid collection on the back of the neck (i.e., the “nuchal translucency”) was also associated with an increased risk for trisomy 21 further revolutionized first-trimester aneuploidy screening.65 First-trimester nuchal translucency measurement alone has a detection rate of 64% to 70% for trisomy 21; however, combining nuchal translucency measurement with PAPP-A and free β-hCG measurement increases that detection rate from 82% to 87% at a 5% fixed FPR.66 This combined approach to first-trimester screening is now routinely offered to all patients, regardless of maternal age, between 10 and 13 6/7 weeks’ gestation.
Combined first-trimester screening can also be used in the detection of patients at high risk for other aneuploidies, including trisomy 18 and trisomy 13. In contrast to trisomy 21, both PAPP-A and free β-hCG levels are decreased in these particular chromosomal abnormalities, while nuchal translucency is increased.67,68 Tul and colleagues67 demonstrated that free β-hCG MoM levels were decreased to <5th percentile of normal in 64% of trisomy 18 cases. Similarly, PAPP-A MoM levels were decreased to <5th percentile of normal in 78% of trisomy 18 cases. Results from the BUN study by Wapner and colleagues69 showed a 90.9% detection rate for trisomy 18 at 2% FPR. Additional studies have demonstrated similar results for combined first-trimester screening with detection rates of 92.3% for trisomy 18 and 88.9% for trisomy 13.70
Adverse Pregnancy Outcomes
In the setting of a normal karyotype, low PAPP-A levels have been found to be associated with multiple adverse pregnancy outcomes. In a secondary analysis of the FASTER trial, Dugoff and colleagues demonstrated that low PAPP-A levels <5th percentile were significantly associated with fetal loss, preterm birth, gestational hypertension, preeclampsia, and low birth weight. Additionally, there was a pattern of an increased magnitude of risk as the PAPP-A level became more extreme. For example, the adjusted odds ratio (aOR) for spontaneous fetal loss <24 weeks was 1.95 (95% CI, 1.44 to 2.62) for PAPP-A levels <10th percentile; however, this risk increased to an aOR of 5.22 (95% CI, 3.09 to 8.80) for PAPP-A levels <1st percentile.71 PAPP-A is derived from the placenta and serves as a protease for insulin-like growth factor (IGF) binding protein-4.72 Decreased levels of PAPP-A are associated with high levels of bound IGF and, subsequently, lower levels of free IGF in the circulation. IGF plays an important role in both fetal growth and trophoblast invasion into the maternal decidua.73,74 Given that low PAPP-A levels are a reflection of low free circulating IGF levels, these observed associations with placenta-mediated adverse pregnancy outcomes appear to be biologically plausible.
Despite these associations, the test performance characteristics of low PAPP-A limit its use as a primary screening tool for adverse pregnancy outcomes. In a population-based cohort study, Krantz and colleagues75 demonstrated that PAPP-A <1st percentile had a sensitivity of 3.3%, specificity of 99.3%, and PPV of 24.1% for fetal growth restriction. Pihl and colleagues76 observed similar test characteristics for PAPP-A <5th percentile for fetal growth restriction, citing a detection rate of only 13% and a PPV of 14%. For preeclampsia, the PPV for PAPP-A <5th percentile is much lower, ranging from 3.5% to 7.6% in prior studies.71,77,78 Incorporating a maternal risk factor-based scoring system to improve the screening efficiency of low PAPP-A for preeclampsia resulted in a sensitivity of 36.4%, specificity of 86.8%, and a positive LR of 2.8. Combining low PAPP-A, African American race, overweight maternal BMI, and maternal history of chronic hypertension and pregestational diabetes resulted in an area under the curve of 0.70, indicating only a modest predictive capability, at best, for the outcome of preeclampsia.79
Consideration of combining low first-trimester PAPP-A levels with elevated second-trimester maternal serum AFP levels has also been given, considering the association between elevated AFP levels and adverse outcomes, as discussed earlier in this chapter. Smith and colleagues80 demonstrated a synergistic effect between low PAPP-A and high AFP in the identification of women at risk to develop fetal growth restriction and preterm birth. Subsequently, Dugoff and colleagues81 demonstrated that the detection rate of patients at risk for early fetal loss <24 weeks was as high as 46% when combining low PAPP-A, high AFP, and low unconjugated estriol, another serum analyte used in second-trimester aneuploidy screening. Unfortunately, the exceedingly small number of patients who undergo sequential screening and actually have this combination of abnormal serum analytes precludes their use as clinical screening tools.
Alternatively, high levels of PAPP-A have not been consistently linked to adverse outcomes. While several studies have suggested a trend toward an increasing risk of macrosomia with high PAPP-A levels, this finding has not been confirmed in subsequent reports.71,82
The association between abnormal levels of first-trimester free β-hCG and adverse pregnancy outcomes has been less consistent. The most commonly cited adverse outcome associated with low free β-hCG in the first trimester has been early pregnancy loss.71,83 Dugoff and colleagues71 demonstrated that free β-hCG levels <1st percentile had a sensitivity of 3.7% at a 1% FPR and a PPV of 2.8% for spontaneous pregnancy loss <24 weeks. Similar to high levels of PAPP-A, high levels of free β-hCG in the first trimester have not been reliably linked to an increased risk for adverse pregnancy outcomes.
On the basis of their overall poor screening efficiency, first-trimester PAPP-A and free β-hCG cannot be recommended as primary screening tools for adverse pregnancy outcome. Additionally, subjecting a population to universal screening for outcomes for which there are currently no proven prevention strategies may lead to increases in fetal surveillance that are neither cost-effective nor clinically indicated. Nevertheless, serial ultrasounds to evaluate fetal growth may be considered in the small number of patients at the highest risk for adverse outcome such as those with PAPP-A levels <1st percentile. Conversely, patients with normal first-trimester PAPP-A and free β-hCG levels can be counseled so that their risk of adverse pregnancy outcome may be reduced.
Ultrasound Evaluation
Patients with an abnormal first-trimester aneuploidy screen who decline invasive prenatal diagnostic testing should undergo a detailed genetic ultrasound during the second trimester to evaluate for major fetal anomalies as well as minor markers of aneuploidy. In patients with an abnormal first-trimester serum screen and a normal karyotype, the protocol for subsequent evaluation is less clear. As opposed to abnormal maternal serum AFP levels, abnormal first-trimester PAPP-A and free β-hCG levels have not been linked to many structural fetal defects. In a 2008 report from a secondary analysis of the FASTER trial, free β-hCG levels >2.0 MoMs were significantly associated with both unilateral and bilateral multicystic dysplastic kidney disease and hydrocele. PAPP-A levels >2.0 MoMs were also associated with hydrocele.84 Targeted fetal renal ultrasound may be considered in this subset of patients.
In an attempt to improve the screening performance of low PAPP-A levels for the prediction of preeclampsia and fetal growth restriction, many authors have proposed the addition of uterine artery Doppler studies.85,86 Poon and colleagues85
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree