Tumour
Mean frequency (%)
Range (%)
Breast
73
47–85
Prostate
68
33–85
Thyroid
42
28–85
Kidney
35
33–40
Lung
36
30–55
Oesophagus
6
5–7
Gastrointestinal
5
3–11
Rectum
11
8–13
The majority of patients with bone metastases develop severe pain as their disease progresses resulting in considerable reduction in quality of life. A multidisciplinary approach to symptom palliation is recommended, tailoring treatment to individual need, the aim of individualised treatment being be “to add life to the years, not years to the life”.
Bone metastases drive different responses in the surrounding bone matrix by intercellular interactions between tumour cells, osteoblasts and osteoclasts. Secretion of PTHrP (PTH related peptide) for example, leads to osteoclast activation, initiating bone destruction after binding to RANKL (receptor for activation of nuclear factor kappa B-ligand) (Ducy et al. 2000). The role of other mediators including interleukins, TNF-α and -β, TGF-α and PGE2 in the development and spread of bone metastases (Averbuch 1993) is being explored.
Bone metastases arising from primary prostate cancer typically elicit an osteoblastic response whereas plasmacytoma and renal cell carcinoma are associated with predominantly destructive bone lesions. Mixed patterns of osteoblastic and osteolytic metastases are more common in breast, lung, colorectal and pancreatic malignancies. Of note, the radiological appearance of an ‘osteoblastic’ or ‘osteolytic’ metastasis is only broadly indicative of the predominant bone reaction within an individual lesion as local bone resorption also exerts a peripheral osteoblastic response (Galasko 1982; Krasnow et al. 1997) that lies below the resolution of plain radiographs.
1 Symptoms and Treatment of Bone Metastases
Approximately, 75 % of patients with bone metastases complain of pain as their main symptom and dominant reason for decreased quality of life (Tannock et al. 1989). Appropriate management may be difficult, particularly where discomfort is poorly localised (Wagner 1984). The prognosis of patients with metastases confined to the skeleton is usually superior to that of patients with soft tissue metasases, in lungs, liver or lymph nodes, for example (Coleman et al. 1998), and therefore merits careful consideration.
In addition to analgesic drugs (prescribed according to the WHO scheme) (World Health Organisation 1990), local external beam radiation therapy and surgical interventions, several radiopharmaceuticals for systemic bone pain palliation have been developed. The first application of the bone-seeking radiopharmaceutical strontium-89 [89Sr]-Chloride was described by Pecher in 1940/1941 (Pecher 1942), followed by the first report of pain palliation in a patient with bone metastases from breast carcinoma using phosphorous-32 [32P] by Friedell (Brucer 1990).
1.1 Radionuclide Treatment
Strontium [89Sr]-chloride, samarium-153-ethylenediamine tetra methylene phosphonic acid [153Sm]-EDTMP and rhenium-186-hydroxyethylidine diphosphonic acid [186Re]-HEDP are approved in Europe for bone pain palliation in patients with bone metastases. [32P] orthophosphate is used in several other countries. 89Sr is a calcium analogue and is incorporated into the newly formed hydroxyapatite of the bone matrix. 153Sm-EDTMP and 186Re-HEDP are radiolabelled bisphosphonates and are adsorbed onto the hydroxyapatite surface of metabolically active bone by the same mechanism as Technetium [99mTc]-labelled bisphosphonates used for diagnostic bone scintigraphy.
Selective uptake depends on the degree of metabolic (i.e. osteoblastic) response elicited in normal bone by the presence of metastatic tissue. Increased bone turnover leads to enhanced incorporation of bone seeking radiopharmaceuticals at metastatic sites by comparison with normal bone and can therefore deliver a high, targeted local radiation dose. Skeletal uptake of the radiolabelled bisphosphonates 186Re-HEDP or 153Sm-EDTMP is in the order of 22 and 48 % of administered activity, respectively (Brenner et al. 2001). The effective half life of 89Sr in bone metastases is > 90 days, compared with 14 days in normal bone (Blake et al. 1986) (Table 2).
Table 2
Physical properties of radiopharmaceuticals used for bone pain palliation
Radionuclide | Carrier | Physical half life [d] | βmax [MeV] | βmean[MeV] | Mean range in tissue [mm] | γ-energy [keV] (%) |
|---|---|---|---|---|---|---|
89Sr | Chloride | 50,5 | 1,46 | 0,583 | 6,7 | – |
153Sm | EDTMP | 1,95 | 0,8 | 0,224 | 3,4 | 103 (Krishnamurthy and Krishnamurthy 2000) |
186Re | HEDP | 3,8 | 1,07 | 0,349 | 4,7 | 137 (Brucer 1990) |
32Pa | Phosphate | 14,28 | 1,71 | 0,695 | 7,9 | – |
188Reb | HEDP | 0,71 | 2,12 | 0,76 | 11,0 | 155 (Ducy et al. 2000) |
117mSnb | DTPA | 13,6 | no β-emission | 0,3 | CE 159 | |
33Pb | Phosphate | 25,34 | 0,249 | 0,85 | 0,05 | – |
32P as sodium phosphate is no longer approved in many countries because of documented myelotoxicity associated with therapeutic administration. More recently, a clinical trial comparing 89Sr and 32P in patients suffering from painful bone metastases from bone metastases reported slightly higher toxicity in the 32P group but comparable efficacy in terms of pain palliation (Fettich et al. 2003). Further research will be necessary to confirm these results particularly in heavily pretreated patients who may have limited bone marrow reserve.
Clinical trials are in progress evaluating the therapeutic potential of other radionuclides for bone pain palliation. These include: tin [117mSn]-DTPA, sodium [33P]-phosphate, rhenium[188Re]-HEDP, lutetium [177Lu]-EDTMP and radium [223 Ra]-chloride.
In addition to clinical variables such as skeletal metastatic burden, disease distribution and prior treatment, myelosuppression resulting from systemic bone seeking radiopharmaceutical therapy reflects effective half life, particle energy and particle range of the radionuclide used in. Reversible Grade 2 and 3 haematological toxicity was demonstrated in 25.5 % of all patients and in 38.7 % of retreated patients using 186Re-HEDP (deKlerk 1995). The use of low-energy beta-emitting radionuclides would be expected to deliver a high absorbed dose to the bone surface but negligible dose to the haematopoietic bone marrow (Bouchet et al. 2000). Theoretical dose calculations predict a three- to sixfold advantage in terms of myelotoxicity risk were 33P to be substituted for 32P, for example (Goddu et al. 2000).
To reduce myelotoxicity, the therapeutic potential of conversion electron emitting radiolabels such as 117mSn-DTPA has been reported. Conversion electrons ejected during the decay of this nuclide have a 1.7–5.5 times lower energy than beta-particles conventionally used for systemic treatment for pain palliation (Hillegonds et al. 2007). But the limiting factor of this compound used in a phase I/II clinical study was not the radiation dose to the marrow but the high amount of DTPA in the current formulation in a 20-fold molar excess over tin (Srivastava 2004). More recently, a new 1:1 chelate was synthesised (Srivastava et al. 2002). Because only few data were published about therapy of cancer patients with 117mSn-DTPA, there is insufficient evidence for recommending this agent now. (Program in evidence-based care—a cancer care Ontario program. Radiopharmaceuticals for the palliation of painful bone metastases. Practical Guideline Report 14–1 (June 2004)).
Estimates of absorbed radiation dose delivered to osteoblastic bone metastases vary widely, ranging from 6 to 61 cGy/MBq for 89Sr, 1,000–14,000 cGy from a standard treatment activity of 1295 MBq 186Re HEDP and a mean dose of 87 Gy from 2,590 MBq 153Sm-EDTMP. A dose of 54 mGy/MBq was reported using 117mSn-DTPA (see next chapter) with bone uptake ranging from 34 to 83 % of injected activity (Atkins et al. 1995).
The bone seeking alpha-particle emitter Radium-223 is predicted to deliver a high radiation absorbed dose to the bone surface with sparing of the bone marrow compartment. Following intravenous administration, skeletal uptake peaks within 1 h of injection with no subsequent redistribution. Phase I and II studies confirm low temporary myelosuppression approximately 4 weeks post treatment, but this rarely exceeds WHO Grade I/II even at high activities [200 kBqkg−1] in heavily pretreated patients (Nilsson et al. 2007).
The mechanism and radiobiology of pain reduction using unsealed source therapy is not understood. A direct radiation effect on neuronal tissue seems unlikely due to the well-known, high radiation resistance of peripheral neurons. It is more conceivable that radiation to cells and tissues surrounding the metastasis promotes cell signalling changes resulting in modulation of both pain reception and transmission. Possible target cells are likely to include macrophages, mast cells, thrombocytes, lymphocytes and endothelial cells which influence secretion of pain mediators such as ATP, histamine, PGE, IL-1 and -2, leukotrienes and substance P.
2 Indications, Contraindications and Procedure of Pain Palliation Treatment
Surgical stabilisation and/or external beam radiation are the treatments of choice for the management of solitary, painful bone metastases, bones at high risk of pathological fracture and in patients with impending spinal cord compression.
Systemic radionuclide therapy is indicated to manage multifocal metastatic bone pain following failure of conventional analgesics and to palliate recurrent pain within a previously irradiated site.
2.1 OR
When the side effects of high dose analgesics become intolerable and significantly compromise quality of life, even if pain control is adequate.
Strontium [89Sr]-Chloride and Rhenium [186Re]-HEDP are approved for pain palliation in patients with bone metastases from prostate cancer, Samarium [153Sm]-EDTMP may also be used in patients suffering from osteoblastic metastases of other tumour types. The activity of 153Sm-EDTMP is adjusted for the patient’s body weight (37 MBq/kg), whereas 89Sr-Chloride and `86Re-HEDP are prescribed as standardised activities [150 and 1295 MBq, respectively].
A prerequisite for radionuclide treatment of metastatic bone pain is the demonstration of focal abnormal skeletal uptake on conventional 99mTc phosphate bone scintigraphy corresponding to known pain sites. Patients should have reasonable bone marrow reserve, as evidenced by (near) normal blood counts. The γ emission of Re-186 and Sm-153 is useful for early posttherapy imaging to confirm selective tracer uptake and appropriate targeting.
Due to the delay between treatment administration and onset of pain relief, which may take 1 week in case of 153Sm-EDTMP or 186Re-HEDP and up to 4 weeks using 89Sr, patients should have a life expectancy of at least 3 months. Absolute contraindications to radionuclide therapy include pregnancy, breast-feeding and severe bone marrow depression, indicated by platelets < 60,000 /μl or leucopenia < 2,400 /μl (Fischer 1999). Acute spinal cord compression, disseminated intravascular coagulation and impaired renal function (urea > 12 mmol/l or creatinine > 150 mmol/l) are regarded as additional contraindications in German, European and American guidelines for pain palliation treatment.
Patients with urinary incontinence should be catheterised prior to treatment to mitigate the risk of radioactive urine contamination. Specialist referral is advised where bones are considered at risk of pathological fracture. To allow time for bone marrow recovery and avoid unpredictable cumulative toxicity, unsealed source treatment should be delayed for 6–8 weeks after completion of chemotherapy. It is recommended that further chemotherapy be deferred for at least 8–12 weeks, depending on the radiopharmaceutical used. A 2–3 month delay is recommended after large-field radiation therapy.
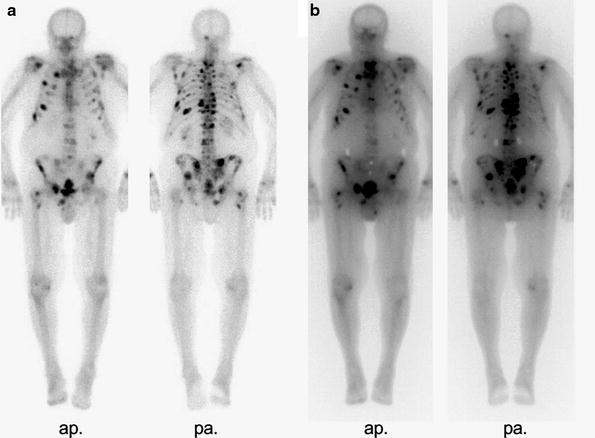
Concomitant treatment with modern bisphosphonates, which are characterised by very low effective levels, does not interfere with the uptake of bone-seeking radionuclides (Lau et al. 2001; McEwan 1994; Marcus et al. 2002). This is contrary to former concerns, regarding the classical drugs Clodronate or Etidronate. Focal abnormal uptake should, however, be confirmed in every patient by pretherapeutic bone imaging (see Fig. 1) as described and correlated with the localisation of pain.
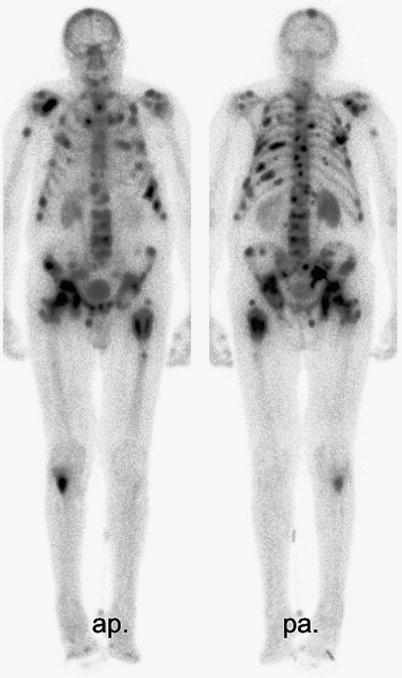

Fig. 1
WBS of a patient with multilocular metastases of a hormone refractory prostate cancer. (3 h.p.i. 700 MBq 99mTc-HDP)
Following appropriate oral hydration, bone-seeking radiopharmaceutical treatment is administered intravenously via a peripheral cannula, usually in an outpatient setting, depending on local legislation. Prior to discharge, the uptake and distribution of activity Re-186-HEDP or Sm-153-EDTMP can be documented by whole body scanning 3 h post injection (see Fig. 2). Renal excretion of the unbound fraction of the radionuclide is very rapid; 45 % of the unbound activity is already excreted 5 h after injection of Re-186-HEDP, 71 % within 3 days (Maxon et al. 1988) compared with, 53 % of the unbound Sm-153-EDTMP excreted via the kidneys within 8 h after injection (Singh et al. 1989).