Blunt Cerebrovascular Injury
7.1 Mechanisms of Vascular Injury
Penetrating trauma is the most common mechanism for cerebrovascular injury, but injuries to the carotid or vertebral arteries from nonpenetrating trauma are more common than previously suspected, with a reported incidence of approximately 1% among those admitted to the hospital for blunt trauma.1,2 The rate of blunt cerebrovascular injury (BCVI) increases with the use of more liberal screening criteria and more sensitive imaging tests,3 indicating there are unrecognized, asymptomatic cases.
Mechanisms of blunt carotid artery injury include direct blows to the neck, intraoral trauma, skull-base fractures involving the carotid canal, severe hyperextension of the neck with contralateral rotation, and hyperflexion.4,5 The distal extracranial portion of the internal carotid artery is particularly vulnerable to injury during extremes of head motion as a result of stretching of the carotid artery over the lateral masses of C1–C3 or compression between the angle of the mandible and upper cervical spine.4
Mechanisms of blunt vertebral artery injury include cervical spinal subluxation, fractures— especially those involving the transverse foramen—and stretch-type injuries caused by lateral bending and axial rotation.6,7 Motor-vehicle accidents, hanging, strangulation, falls from a height, and sports accidents are frequent causes of BCVI, but injuries also occur in association with seemingly minor trauma, such as chiropractic manipulations. It is controversial whether cerebrovascular injuries occurring with minor trauma are best classified as BCVI or whether these injuries represent part of a spectrum of “spontaneous” cervical artery dissections that may have a different underlying pathophysiology.8 Additionally, gunshot wounds, although typically associated with penetrating vascular injuries, may also cause BCVI at sites remote from the bullet tract, presumably secondary to extreme neck movements.9
7.2 Rationale for Screening
Cerebrovascular injuries from blunt trauma are more common than once suspected, and the outcomes are frequently devastating, with a high risk of stroke and associated morbidity and mortality. Many patients with BCVI are initially asymptomatic, but platelet aggregation and thrombosis at the site of an intimal injury can lead to thromboembolic stroke after a delay of hours to days,10 or even months to years.5,11 Accumulating class II and class III evidence indicates that early institution of anticoagulant or antiplatelet therapy reduces the risk of stroke in asymptomatic patients with BCVI and improves neurologic outcomes in symptomatic patients.12–16 Accordingly, it is crucial to identify those patients at high risk for clinically occult BCVI who can be screened with vascular imaging and treated before they develop strokes.
7.3 Indications for Vascular Imaging in Blunt Trauma
Blunt trauma victims require vascular imaging when they have signs or symptoms of BCVI or when they have high risk factors for BCVI, based on the mechanism of injury or associated injury patterns.
7.3.1 Signs and Symptoms
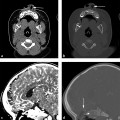
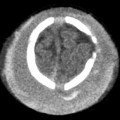
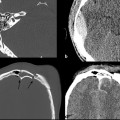
Trauma patients with signs or symptoms attributable to BCVI require emergent diagnostic imaging and prompt treatment of hemorrhages or strokes. Signs and symptoms of BCVI include the following: (1) hemorrhage from the mouth, nose, ears, or face of potential arterial origin; (2) large or expanding cervical hematoma; (3) cervical bruit in a patient under age 50; (4) unexplained focal or lateralizing neurologic deficit, including hemiparesis, transient ischemic attack, vertebrobasilar insufficiency, or Horner syndrome; (5) acute infarct on computed tomography (CT) or magnetic resonance imaging (MRI); and 6) any neurologic deficit inconsistent with CT or MRI findings.17
7.3.2 Risk Factors
Relatively inclusive indications for screening of asymptomatic patients were established by the Denver group to identify a larger number of at-risk patients.13,17–19 The Denver Imaging Criteria are based on injury mechanisms combined with high-risk radiologic or physical examination findings.
These criteria include an injury mechanism capable of producing severe cervical hyperextension with rotation or hyperflexion associated with any of the following: complex mandibular fractures, displaced Le Fort type II or III midface fractures, skull-base fractures involving the carotid canal, closed head injuries with diffuse axonal injury and low GCS score, cervical subluxations or fractures extending to the transverse foramen, any fracture at C1–C3, near-hanging resulting in cerebral anoxia, and seat-belt abrasions with severe swelling or altered mental status. The definition of complex mandible fracture has not been clarified.
Subsequent logistic regression analysis identified four independent predictors of carotid artery injury: (1) GCS of 6 or lower, (2) petrous temporal bone fracture, (3) diffuse axonal injury, and (4) Le Fort II or III fractures.19 A similar analysis showed cervical spine fractures to be an independent predictor of vertebral artery injury.19 These “high-risk” factors constitute a more restrictive core set of screening criteria, but approximately 20% of the patients screened using the more liberal preceding criteria had none of the independent risk factors. Additionally, a substantial number of cerebrovascular injuries occur in patients who have no associated injuries or classic risk factors and are diagnosed either incidentally or because screening was performed based on clinical suspicion of injury.18–22 Accordingly, continued use of broad screening criteria is likely justified to prevent missing treatable injuries.
Because screening is now usually performed noninvasively using CT angiography (CTA), some favor using even broader screening criteria. In patients with an appropriate mechanism of injury, additional proposed criteria include any fracture of the mandible or skull base;22,23 any cervical spine fracture2,23 or thoracolumbar spine fracture;2 any major thoracic trauma;2,20 any thoracic injury combined with head trauma or any thoracic vascular injury;22 scalp degloving injuries;22and gunshot wounds to the head, face, or neck.9 Further research is needed to determine the optimal screening criteria to detect the patients not captured by the Denver criteria, as well as to balance the risks and cost of screening with the benefits.
7.4 Screening Modality of Choice
The optimal imaging modality for screening remains another important topic for ongoing investigations. Conventional catheter-based four-vessel digital subtraction angiography (DSA) is the gold standard for diagnosis of BCVI, but it is invasive and consumes time and resources. Duplex ultrasonography, magnetic resonance angiography (MRA), and CTA are noninvasive diagnostic imaging modalities that have been considered as potential alternatives to DSA.
Ultrasound, although useful for screening the extracranial carotid arteries for atherosclerotic disease, suffers from marked limitations in evaluating the carotid arteries near the skull base, where most carotid injuries occur. Ultrasound is also limited in evaluating the vertebral arteries within the bony vertebral foramen, where most vertebral artery injuries occur. In a large retrospective series of more than 1400 blunt trauma patients screened with ultrasound, eight injuries were missed that resulted in symptomatic neurologic deficits, and the overall sensitivity of the test was only 39%.24 Ultrasound is therefore not recommended for screening.16,17
Although MRA initially held promise as a noninvasive option for screening, in the two series that prospectively compared MRA with DSA, MRA had a sensitivity of only 50 to 75% and a specificity of only 47 to 67%.25,26 MRA also has practical disadvantages, including long examination times and lack of availability at some centers, and MRA may not be feasible in unstable trauma patients requiring external monitoring equipment. MRA is therefore not recommended for screening at this time.16,17 MRA may still be an option in patients with contraindications to iodinated contrast or for follow-up of BCVI.
Axial fat-saturated proton density or T1-weighted images are useful for demonstrating hyperintense intramural hematoma in subacute dissections, but they are less useful during the acute phase when the signal characteristics of intramural hematoma are inconspicuous. Special techniques such as high-resolution vessel-wall black blood MRI with dedicated neck coils may allow for improved performance of MRI or MRA in the future for diagnosing BCVI.
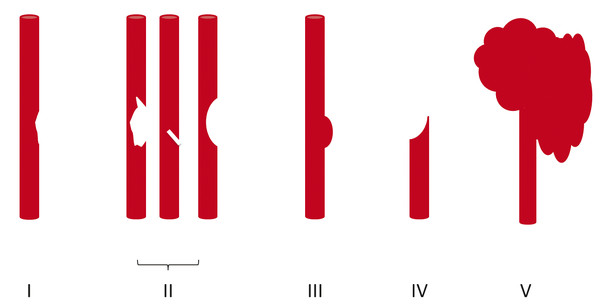
Fig. 7.1 Schematic depicting Denver Grading Scale for blunt cerebrovascular injury (see ▶ Table 7.1 for description).
Early studies comparing CTA with DSA demonstrated poor sensitivity and specificity of CTA, but with the advent of multislice detector techniques, CTA has emerged as the screening modality of choice.17,27–31 CTA has the advantages of being fast, noninvasive, and readily available. Moreover, most trauma patients already require CT scanning. Some controversy remains concerning the accuracy of CTA compared with DSA, with relatively poor sensitivity and specificity reported at some institutions.32 It has been suggested that some of the variability in the reported accuracy of CTA reflects differences in radiologist experience, subspecialty training, or interpretive abilities.21,31,33 For example, in the study by Malhotra and colleagues, all the false-negative CTA interpretations occurred in the first half of the study period, indicating that interpretive ability improved with experience.34 Additionally, lower-grade injuries are more difficult to detect on CTA compared with DSA,25 so CTA will have a lower sensitivity in population samples with lower-grade injuries. Strategies for improving the detection of subtle findings on CTA include evaluating each of the four vessels individually, as one would with DSA, and using both source images and multiplanar reconstructions to carefully examine the most commonly injured areas, such as near the skull base and adjacent to fractures of the carotid canals or transverse foramen.31
7.5 Classification of Arterial Injuries
The Denver Grading Scale (▶ Fig. 7.1; ▶ Table 7.1) has been widely adopted for classifying traumatic cerebrovascular injuries based on the angiographic appearance of the injured vessel.18 Grade I injuries are defined as irregularity of the vessel wall or a dissection or intramural thrombus causing less than 25% narrowing of the vessel lumen (▶ Fig. 7.2). Grade II injuries are defined as a dissection or intramural thrombus causing luminal stenosis of 25% or greater (▶ Fig. 7.3a,b), or injuries in which a raised intimal flap (▶ Fig. 7.3c) or an intraluminal thrombus (▶ Fig. 7.4) occurs. Grade II injuries also include small, hemodynamically insignificant arteriovenous fistulae.35
| Injury Grade | Description |
| I | Vessel wall irregularity or dissection/intramural hematoma with <25% luminal stenosis |
| II | Intraluminal thrombus or raised intimal flap or dissection/intramural hematoma with ≥25% luminal stenosis |
| III | Pseudoaneurysm |
| IV | Occlusion of a vessel |
| V | Vessel transection with free contrast extravasation or hemodynamically significant arteriovenous fistula |
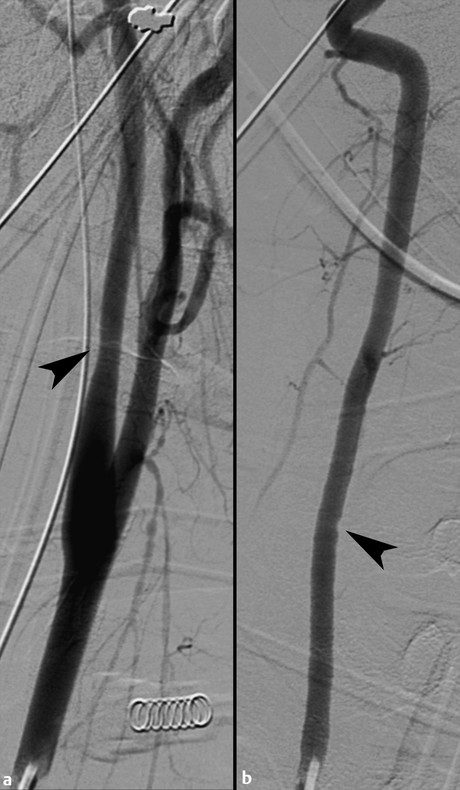
Fig. 7.2 Grade I injuries. (a) Left proximal internal carotid artery focal smooth narrowing of less than 25% just distal to the carotid bulb (arrowhead). No visible intimal flap or pseudoaneurysm. (b) Right vertebral artery focal wall irregularity at the level of C6–C7 (arrowhead), without stenosis, intimal flap, or pseudoaneurysm.
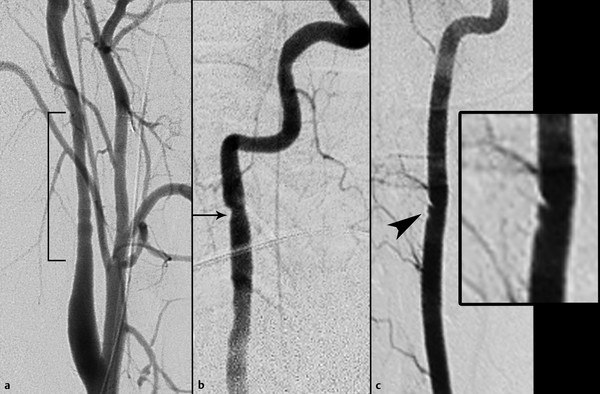
Fig. 7.3 Grade II injuries. (a) Right proximal internal carotid artery long-segment wall irregularity with narrowing of 50% (bracket). (b) Right vertebral artery focal-wall irregularity with narrowing of 40% (arrow
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree