Tumors of neuroepithelial tissue
Tumors of the pineal region
Angiolipoma
8861/0
Astrocytic tumors
Pineocytoma
9361/1
Hibernoma
8880/0
Pilocytic astrocytoma
9421/1a
Pineal parenchymal tumor of intermediate differentiation
9362/3
Liposarcoma
8850/3
Pilomyxoid astrocytoma
9425/3 b
Pineoblastoma
9362/3
Solitary fibrous tumor
8815/0
Subependymal giant cell astrocytoma
9384/1
Papillary tumor of the pineal region
9395/3 b
Fibrosarcoma
8810/3
Pleomorphic xanthoastrocytoma
9424/3
Embryonal tumors
Malignant fibrous histiocytoma
8830/3
Diffuse astrocytoma
9400/3
Medulloblastoma
9470/3
Leiomyoma
8890/0
Fibrillary astrocytoma
9420/3
• Desmoplastic/nodular medulloblastoma
9471/3
Leiomyosarcoma
8890/3
Gemistocytic astrocytoma
9411/3
• Medulloblastoma with extensive nodularity
9471/3 b
Rhabdomyoma
8900/0
Protoplasmic astrocytoma
9410/3
• Anaplastic medulloblastoma
9474/3 b
Rhabdomyosarcoma
8900/3
Anaplastic astrocytoma
9401/3
• Large cell medulloblastoma
9474/3
Chondroma
9220/0
Glioblastoma
9440/3
CNS primitive neuroectodermal tumor
9473/3
Chondrosarcoma
9220/3
Giant cell glioblastoma
9441/3
• CNS neuroblastoma
9500/3
Osteoma
9180/0
Gliosarcoma
9442/3
• CNS ganglioneuroblastoma
9490/3
Osteosarcoma
9180/3
Gliomatosis cerebri
9381/3
• Medulloepithelioma
9501/3
Osteochondroma
9210/0
Oligodendroglial tumors
• Ependymoblastoma
9392/3
Hemangioma
9120/0
Oligodendroglioma
9450/3
Atypical teratoid/rhabdoid tumor
9508/3
Epithelioid hemangioendothelioma
9133/1
Anaplastic oligodendroglioma
9451/3
Tumors of cranial and paraspinal nerves
Hemangiopericytoma
9150/1
Oligoastrocytic tumors
Schwannoma (neurilemoma, neurinoma)
9560/0
Anaplastic hemangiopericytoma
9150/3
Oligoastrocytoma
9382/3
• Cellular
9560/0
Angiosarcoma
9120/3
Anaplastic oligoastrocytoma
9382/3
• Plexiform
9560/0
Kaposi sarcoma
9140/3
Ependymal tumors
• Melanotic
9560/0
Ewing sarcoma—PNET
9364/3
Subependymoma
9383/1
Neurofibroma
9540/0
Primary melanocytic lesions
Myxopapillary ependymoma
9394/1
• Plexiform
9550/0
Diffuse melanocytosis
8728/0
Ependymoma
9391/3
Perineurioma
Melanocytoma
8728/1
• Cellular
9391/3
Perineurioma, NOS
9571/0
Malignant melanoma
8720/3
• Papillary
9393/3
Malignant perineurioma
9571/3
Meningeal melanomatosis
8728/3
• Clear cell
9391/3
Malignant peripheral nerve sheath tumor (MPNST)
Other neoplasms related to the meninges
• Tanycytic
9391/3
Epithelioid MPNST
9540/3
Hemangioblastoma
9161/1
Anaplastic ependymoma
9392/3
MPNST with mesenchymal differentiation
9540/3
Lymphomas and hematopoietic neoplasms
Choroid plexus tumors
Melanotic MPNST
9540/3
Malignant lymphomas
9590/3
Choroid plexus papilloma
9390/0
MPNST with glandular differentiation
9540/3
Plasmacytoma
9731/3
Atypical choroid plexus papilloma
9390/1
Tumors of meningothelial cells
Granulocytic sarcoma
9930/3
Choroid plexus carcinoma
9390/3
Meningioma
9530/0
Germ cell tumors
Other neuroepithelial tumors
• Meningothelial
9531/0
Germinoma
9064/3
Astroblastoma
9430/3
• Fibrous (fibroblastic)
9532/0
Embryonal carcinoma
9070/3
Chordoid glioma of the third ventricle
9444/1
• Transitional (mixed)
9537/0
Yolk sac tumor
9071/3
Angiocentric glioma
9431/1
• Psammomatous
9533/0
Choriocarcinoma
9100/3
Neuronal and mixed neuronal–glial tumors
• Angiomatous
9534/0
Teratoma
9080/1
Dysplastic gangliocytoma of cerebellum
9493/0
• Microcystic
9530/0
• Mature
9080/0
Desmoplastic infantile astrocytoma/ganglioglioma
9412/1
• Secretory
9530/0
• Immature
9080/3
Dysembryoplastic neuroepithelial tumor
9413/0
• Lymphoplasmacyte rich
9530/0
• Teratoma with malignant transformation
9084/3
Gangliocytoma
9492/0
• Metaplastic
9530/0
Mixed germ cell tumor
9085/3
Ganglioglioma
9505/1
• Chordoid
9538/1
Tumors of the sellar region
Anaplastic ganglioglioma
9505/3
• Clear cell
9538/1
Craniopharyngioma
9350/1
Central neurocytoma
9506/1
• Atypical
9539/1
Adamantinomatous
9351/1
Extraventricular neurocytoma
9506/1 b
• Papillary
9538/3
Papillary
9352/1
Cerebellar liponeurocytoma
9506/1 b
• Rhabdoid
9538/3
Granular cell tumor
9582/0
Papillary glioneuronal tumor
9509/1 b
• Anaplastic (malignant)
9530/3
Pituicytoma
9432/1b
Rosette-forming glioneuronal tumor of the fourth ventricle
9509/1 b
Mesenchymal tumors
Spindle cell oncocytoma of the adenohypophysis
8291/0b
Paraganglioma
8680/1
Lipoma
8850/0
It is important to remark that CNS tumors do not currently have TNM designations [4]. Attempts at developing a TNM-based classification and staging system for CNS tumors have been disappointing. There are multiple reasons for this. (1) Tumor size is significantly less relevant than tumor histology and tumor grading (Table 9.2). Thus, the location of the tumor, so the T classification, is less pertinent than the biologic nature of the tumor itself. (2) Since the brain and spinal cord have no lymphatics, the N classification does not apply. (3) An M classification is not pertinent to the majority of CNS tumors, because they have tendency to recur locally rather than disseminating at distance.
Table 9.2
WHO grades of CNS tumors
I | II | III | IV | I | II | III | IV | ||
|---|---|---|---|---|---|---|---|---|---|
Astrocytic tumors | Central neurocytoma | ● | |||||||
Subependymal giant cell astrocytoma | ● | Extraventricular neurocytoma | ● | ||||||
Pilocytic astrocytoma | ● | Cerebellar liponeurocytoma | ● | ||||||
Pilomyxoid astrocytoma | ● | Paraganglioma of the spinal cord | ● | ||||||
Diffuse astrocytoma | ● | Papillary glioneuronal tumor | ● | ||||||
Pleomorphic xanthoastrocytoma | ● | Rosette-forming glioneuronal tumor of the fourth ventricle | ● | ||||||
Anaplastic astrocytoma | ● | Pineal tumors | |||||||
Glioblastoma | ● | Pineocytoma | ● | ||||||
Giant cell glioblastoma | ● | Pineal parenchymal tumor of intermediate differentiation | ● | ● | |||||
Gliosarcoma | ● | Pineoblastoma | ● | ||||||
Oligodendroglial tumors | Papillary tumor of the pineal region | ● | ● | ||||||
Oligodendroglioma | ● | Embryonal tumors | |||||||
Anaplastic oligodendroglioma | ● | Medulloblastoma | ● | ||||||
Oligoastrocytic tumors | CNS primitive neuroectodermal tumor (PNET) | ● | |||||||
Oligoastrocytoma | ● | Atypical teratoid/rhabdoid tumor | ● | ||||||
Anaplastic oligoastrocytoma | ● | Tumors of the cranial and paraspinal nerves | |||||||
Ependymal tumors | Schwannoma | ● | |||||||
Subependymoma | ● | Neurofibroma | ● | ||||||
Myxopapillary ependymoma | ● | Perineurioma | ● | ● | ● | ||||
Ependymoma | ● | Malignant peripheral nerve sheath tumor (MPNST) | ● | ● | ● | ||||
Anaplastic ependymoma | ● | Meningeal tumors | |||||||
Choroid plexus tumors | Meningioma | ● | |||||||
Choroid plexus papilloma | ● | Atypical meningioma | ● | ||||||
Atypical choroid plexus papilloma | ● | Anaplastic/malignant meningioma | ● | ||||||
Choroid plexus carcinoma | ● | Hemangiopericytoma | ● | ||||||
Other neuroepithelial tumors | Anaplastic hemangiopericytoma | ● | |||||||
Angiocentric glioma | ● | Hemangioblastoma | ● | ||||||
Chordoid glioma of the third ventricle | ● | Tumors of the sellar region | |||||||
Neuronal and mixed neuronal–glial tumors | Craniopharyngioma | ● | |||||||
Gangliocytoma | ● | Granular cell tumor of the neurohypophysis | ● | ||||||
Ganglioglioma | ● | Pituicytoma | ● | ||||||
Anaplastic ganglioglioma | ● | Spindle cell oncocytoma of the adenohypophysis | ● | ||||||
Desmoplastic infantile astrocytoma and ganglioglioma | ● | ||||||||
Dysembryoplastic neuroepithelial tumor | ● |
Neuroepithelial tissue tumors are by far the most common form of brain tumors. They are commonly referred to as gliomas. There are three types of gliomas, namely astrocytomas, oligodendrogliomas, and ependymomas. Gliomas are by far the most common and more ominous brain tumor type. Tumors deriving from the meninges, i.e., meningiomas, are another common form of CNS tumors. Less frequent histotypes include embryonal tumors, pineal gland tumors, choroids plexus tumors, ependymal tumors, and tumors of the cranial and paraspinal nerves.
On the basis of histological features, CNS tumors are divided into low-grade and high-grade tumors. Grading is based on the degree of nuclear atypia, mitosis, microvascular proliferation, and necrosis. Grading is clinically important because prognosis worsens as tumor grading increases. Tumor grade is determined by multiple histological sampling of tumor tissue. Low-grade gliomas are grade I and II, while high-grade gliomas are grade III and IV. Since histological features may vary within different areas of the same tumors, the most malignant region determines the grade of the tumor. Oligodendrogliomas, ependymomas, and astrocytomas can be either grade II or grade III, depending on the histological features. Grade III astrocytomas are commonly referred to as anaplastic astrocytomas. Glioblastoma, a grade IV glioma, is the most malignant as well as the most common form of gliomas, accounting for about 50% of all gliomas [5]. Glioblastomas are exceptionally aggressive tumors that are generally resistant to treatment. Average survival is less than 1 year from diagnosis. Glioblastomas are characterized by hypercellular anaplastic glioma cells with marked mitotic activity as well as necrosis and endothelial proliferation. Neoplastic cells frequently reveal pleomorphism showing different histological features such as small homogeneous cells with scant cytoplasm, fibrillary-shaped cells, multinucleated giant cells, and cells with pleomorphic nuclei and cytoplasm [6]. Because of such cellular pleomorphism, the tumor is often referred to as glioblastoma multiforme (GBM) [6]. Histological features in glioblastomas are associated with specific genetic alterations and with clinical outcome: the presence of necrosis is associated with poor survival, while the presence of oligodendroglial components is predictive of longer survival [7]. Amplification of the epithelial growth factor receptor (EGFR) is associated with necrosis and worse outcome [6].
Recurrence of disease is frequent in brain tumors. Recurrent gliomas are termed secondary gliomas. Clinical observations and biological studies differentiate primary and secondary gliomas. Primary gliomas develop without clinical or histological evidence of a less malignant precursor lesion. They represent the majority of cases (>90%) and they affect mainly elderly patients. Primary gliomas are genetically well characterized. The most common genetic alteration is PTEN (phosphatases and tensin homology) gene, which encodes a central domain that is important in the function of phosphatases. Secondary glioblastomas develop through progression from a low-grade diffuse astrocytoma to anaplastic astrocytoma and present in younger patients. Evolution of a recurrent high-grade glioblastoma is more rapid than a primary high-grade glioblastoma. In the pathway to secondary high-grade glioblastoma, TP53 mutations are the most frequent and earliest detectable genetic alteration, already present in 60% of precursor low-grade astrocytomas [8].
Clinical Presentation
Symptoms of brain tumors depend on location, size, and rate of growth of the tumor rather than on histological type. High-grade, fast growing tumors have early symptom onset, while slow-growing, small-sized tumors may remain asymptomatic for many years, especially if they are located in cerebral mute areas, such as the frontal lobe. In these cases, brain neoplasms can be an incidental finding at computed tomography (CT) or magnetic resonance (MR) imaging.
Symptoms can be either focal or generalized. Generalized symptoms reflect increased intracranial pressure. The most common symptom is headache. Typically, the headache is diffuse, but it may affect only one side, frequently reflecting the site of the tumor. Generally, headache is more intense on awakening in the morning and responds poorly to analgesics; it wanes spontaneously after few hours. Rarely, headache is throbbing and mimicking migraine. Nausea, vomiting, and a sixth-nerve palsy are less common.
Focal symptoms or signs take place when the neoplasm compresses the nearby cerebral parenchyma. They can be motor, sensory, or sensory–motor. Aphasia is typical of frontal lobe involvement, ataxia is typical of cerebellar involvement, and diplopia occurs with optic nerve gliomas. Seizures occur at variable degree in brain tumor patients, being more frequent in low-grade tumors. Typically, the seizures are focal but they may become generalized and cause loss of consciousness.
The Clinical Problem
Before entering into the discussion of PET and SPECT imaging of brain tumors, the clinical situations in which PET/SPECT may be useful are anticipated (Table 9.3).
Table 9.3
Clinical indications to SPECT or PET imaging in gliomas
Pre-therapy |
1.Diagnosis |
2.Noninvasive tumor grading |
3.Guidance for stereotactic biopsy |
4.Surgical planning |
5.Identification of metabolically active tumor (or biological target volume, BTV) for radiotherapy planning |
6.Evaluation of multidrug resistance |
Post-therapy |
1.Identification of residual tumor |
2.Differential diagnosis between tumor recurrence and radiation necrosis |
3.Assessment of response to therapy |
4.Prediction of survival |
Diagnosis
CNS masses may be incidental findings on MR or CT examinations performed as part of diagnostic staging for non-CNS pathology, or following neuroradiological examinations performed for neurological or psychiatric symptoms. In most cases, neuroradiological studies are sufficient for the diagnosis. PET may be useful to reinforce the diagnostic hypothesis and to address other issues for the presurgical evaluation.
Noninvasive Tumor Grading
Grading is obtained following histological analysis of stereotactic biopsy or of the surgical specimen, if biopsy is not possible. Thus, it would be desirable to predict tumor grade noninvasively with brain imaging or to use imaging technique to guide biopsy to the areas that are more likely to be higher grade.
Guidance for Stereotactic Biopsy
A typical feature of gliomas is the variability in histological grade that can occur within the same tumor. This is particularly common in high-grade tumors that derive from low-grade forms. More than 60% of tumors contain both low-grade and high-grade features. As a consequence, sampling errors on stereotactic biopsy are a matter of concern. MR and CT do not provide sufficiently accurate information for distinguishing low-grade from high-grade areas. Whether or not PET can be used for guiding biopsy sampling has been addressed in various studies.
Surgical Planning
Conventional MR imaging may underestimate the tumor extent in cerebral gliomas. In fact, edges of the abnormal MR lesion in the autopsy specimens of brains affected by gliomas may have viable tumor cells on histopathological examination. Accurate delineation of tumor extent is critical for a complete resection and for localizing metabolically active areas within the tumor in case of a partial resection.
Radiotherapy Planning
Tumor delineation is also important to define the tumor volume that must be irradiated for therapeutic purposes. Adjuvant radiation therapy improves tumor control and is therefore carried out in all tumor patients. Inaccurate tumor delineation with persistence of viable tumor cells beyond the target area (i.e., geographic misses) is a major cause of treatment failure.
Radiotherapy planning volume was traditionally based on CT and MR. The target volume defined on the basis of morphological imaging is referred to as gross tumor volume (GTV). One of the most important limitations in the application of image-guided radiotherapy consists in the failure to define the tumor extension precisely, which can be attributed to nonspecific changes of MR and CT after surgery or radiation therapy. Thus, PET has been used to identify the metabolically active brain areas. The metabolically active volume, as identified by PET, is referred to as biological target volume (BTV). Logical union of GTV and BTV provides the ultimate planning target volume (PTV).
Differentiation of Tumor Recurrence Versus Radiation Injury
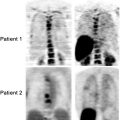
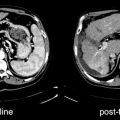
This is currently the most common indication for a PET study. Radiotherapy is associated with a risk of radiation necrosis, especially in the case of tridimensional, conformal treatments that deliver high local doses to small volumes. The occurrence of necrosis is difficult to predict, and it is necessary to differentiate it from tumor recurrence. Radiotherapy-induced injury of the blood–brain barrier (BBB) and brain tissue causes edema, so that contrast enhancement may be evident on both CT and MR. Tumor recurrence is also characterized by contrast enhancement. This typically occurs for high-grade gliomas that invade cerebral vessels, thereby increasing BBB permeability. Thus, simple observation of contrast enhancement on CT and MR requires further differential diagnosis.
Assessment of Response to Therapy
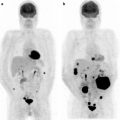
The majority of non-CNS cancers are responsive to some lines of chemotherapy, and evaluation of response to therapy is a cornerstone of oncology imaging. Instead, CNS tumors respond poorly to traditional chemotherapies, and metabolic changes induced by chemotherapy are not routinely evaluated. SPECT and PET studies were performed to assess the biological basis of the poor chemosensitivity of gliomas to most common chemotherapy drugs. Nevertheless, the recent development of new chemotherapy agents, such as Temozolomide, has renovated interest in assessing the response to treatment. PET has also been used to address the effect on cerebral blood flow (CBF) and glucose metabolism in response to dexamethasone, which is generally used to reduce edema and to alleviate neurological symptoms.
Prognosis
By assessing the neural function, PET may be useful to predict the behavior of brain tumors. Such prognostic information may be obtained at any time point, i.e., initial staging, response to therapy, and restaging.
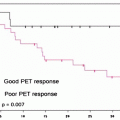
This classification of clinical indications to brain tumor imaging is “problem-oriented.” Not always is a PET scan requested to address all these issues, and some of them are strictly correlated. Using temporal criteria in relation to the main therapeutic events and very similarly to what is done in general oncology, PET scans are indicated in patients with brain tumors: (1) for initial staging; (2) after surgery for evaluation of residual mass; (3) for restaging, after a clinical or instrumental suspicion of recurrence of disease, or for differential diagnosis from radiation necrosis; and (4) for the evaluation of response to therapy.
Because of the concern for possible false-positive findings due to nonspecific BBB disruption, PET scans are not indicated during follow-up if patients are asymptomatic or no abnormal findings are detected on MR. The discussion on PET/SPECT imaging of brain tumors with different radiopharmaceuticals will try to merge both criteria. Clinical problems 1–5 are addressed at initial staging. Tumor recurrence versus radiation necrosis is the typical restaging situation. Prognostic information can be obtained with appropriate study designs at any time point.
Prognosis
There are several prognostic factors for brain tumors (Table 9.4).
Table 9.4
Prognostic factors in CNS tumors
Histology |
Pathologic grade and accuracy of diagnosis |
Presence and extent of necrosis |
Presence of gemistocytes |
Proliferative fraction (Ki-67) |
Presence of oligodendroglial component |
Presence or absence of cells in mitosis, endothelial proliferation |
Age of patient |
Functional neurological status |
Karnofsky Performance Score |
Symptom presentation and duration before diagnosis |
Presentation with seizure and long duration are favorable prognostic factors |
Location of tumor |
Unifocal or multifocal |
Primary or recurrent tumor |
Extent of resection |
Biopsy, subtotal, radical removal |
Metastatic spread |
CNS or extraneural |
Patterns of enhancement on imaging studies |
Molecular aspects |
1 p, 19 q definitions |
MGMT methylation |
Tumor Histology
The histology of tumors that affect the brain and spinal cord is by far the most important variable affecting prognosis, and in many cases it determines the treatment modalities that are employed. Prognosis is worst with highest grade. The latest WHO classification system has combined tumor nomenclature with an associated grading system so that the actual histological diagnosis directly correlates with the histological grade of the tumor. The most common histologies for brain and spinal cord tumors are given in Tables 9.1 and 9.2, along with the tumor grade for each different diagnostic category.
Age
Most retrospective outcome studies of brain tumor therapy show that older age at the time of diagnosis is a powerful predictor of poor outcome [9, 10]. This fact holds true for the gliomas, which are the most common primary brain tumors, and for most other tumors that affect the adult population, including most metastatic tumors to the brain. There are, however, some childhood tumors that have a very poor prognosis, are inherently high grade, and rapidly progress to a fatal outcome. Some metastatic tumors, such as melanoma, occur in younger patients and also violate this general statement with regard to the specific effect of age on prognosis.
Extent of Tumor Resection
In patients who are treated surgically for tumors of the CNS, the extent of resection is often directly correlated with the outcome. This is a less powerful predictor than tumor histology or age, but most retrospective studies confirm that extent of removal is positively correlated with survival. For this reason, documentation of whether a surgical tumor removal is “gross total,” “subtotal,” or “biopsy only” is useful in determining future therapy and prognosis and ideally is accompanied by MR-based quantitative assessment.
Tumor Location
Because of the different importance of various areas of the brain, the location of a given tumor affecting the brain can have a major impact on the functional outcome, survival, and nature of therapy.
Functional Neurologic Status
Another important prognostic factor in most retrospective studies of CNS tumors is the functional neurologic status of the patient at the time of diagnosis [7]. This has been estimated traditionally using the Karnofsky Performance Scale, which is reproducible, is well known by most investigators, and is in common use for stratification of patients entering clinical trials for the treatment of brain tumors. The outcome and prognosis of patients correlate fairly well with functional neurological status.
Metastatic Spread
Tumors affecting the CNS rarely develop extraneural metastases because of inherent biologic characteristics of these tumors and because the brain does not have a well-developed lymphatic drainage system. In addition, many patients with tumors of the CNS have a short life expectancy, which further limits the likelihood of metastatic spread. Certain tumors do spread through cerebrospinal fluid (CSF) pathways, and such spread has a major impact on survival. Dissemination through the CSF pathway is a hallmark of certain childhood tumors, e.g., primitive neuroectodermal tumors, many of which carry a poor prognosis; this phenomenon, however, is rarely seen in adult patients with the more common CNS tumors. Primary lymphomas of the CNS may spread along the craniospinal axis and sometimes exhibit intraocular dissemination.
Therapy of CNS Tumors
Primary brain tumors have a dismal prognosis. Five-year survival rates can be as low as <5% for glioblastoma, 30% for astrocytomas, but up to 100% for benign neoplasms as meningiomas. These rates have remained virtually unchanged over the last years, thus indicating the lack of substantial improvement in prognosis in spite of technological evolutions in diagnostic and surgical strategies [5].
Therapy is generally a combination of surgery, chemotherapy, and radiotherapy or radioimmunotherapy. Because of the limited efficacy and potentially severe side effects of each treatment, many studies are in progress to enhance the efficacy of this multimodality approach.
Surgery
Surgery remains the primary therapeutic approach for most brain tumors. The goal of surgery is twofold. First, it is necessary for accurate histological diagnosis of the tumor. Second, it allows tumor debulking, which is necessary for preventing or alleviating symptoms. Based on the extent, resections can be classified as either partial or complete. Partial resections are performed for large, deeply infiltrating neoplasms for which only debulking is possible or for neoplasms located near difficult to access regions. Complete resection is otherwise preferred. However, except for pilocytic astrocytomas, the necessary extent of surgical resection beyond obtaining a tissue diagnosis remains controversial. Although a larger resection has greater curative potentials, the surgeon always faces a balanced compromise between the likelihood of increasing the local control and the risk of causing important side effects.
Radiation Therapy
Radiation therapy has been used extensively in the treatment of malignant and aggressive intracranial tumors, and its important role has repeatedly been validated by prolonged patient survival rates and increased tumor control. In fact, radiotherapy remains the single, most effective adjuvant therapy after surgery. As more modern approaches are employed for surgery and radiotherapy, attention is being directed to the usefulness of radiation as either primary or secondary treatment of benign tumors. Specifically, primary treatment encompasses irradiation of small benign tumors without biopsy confirmation of tumor type; secondary treatment involves postoperative radiation therapy [11].
In completely or partially resected low-grade astrocytomas, radiation therapy can often be deferred until there are signs of progressive recurrence or malignant transformation. Although the 5-year progression-free survival is improved with immediate irradiation, the overall 5-year overall survival is unchanged. Since radiation therapy can cause significant cognitive and pituitary dysfunction in longer term survivors, well-differentiated astrocytomas can be treated with a lower dose [12].
In the case of GBM, radiotherapy is complicated by the diffuse infiltrative nature of the disease and by its extreme radioresistance. However, a clear survival advantage of post-resection radiation has been ascertained by randomized trials, showing that the median progression-free survival of GBM patients improved from 6 months to 10 to 12 months following near-maximal brain-tolerated doses of ionizing radiation [2, 12].
The use of radiation therapy for brain tumors has continuously increased over the last years due to the development of highly sophisticated techniques, such as stereotactic radiosurgery and three-dimensional, image-guided radiotherapy, which allow for a highly precise delivery of the radiation dose to the target volume.
Chemotherapy
The overall efficacy of chemotherapy in brain tumors is quite limited. First, the BBB limits access to the brain to molecules with low molecular weight and high lipophilicity, thereby preventing CNS access to a wide range of drugs. Second, another major obstacle to reach optimal chemotherapeutic outcomes is the fact that cancer cells develop multidrug resistance towards a broad spectrum of structurally unrelated cytotoxic drugs that have different modes of action. Many genes have been implicated in the development of multidrug resistance (MDR) in gliomas, including the MDR1 gene encoding for the P-glycoprotein, an ATP-dependent drug efflux pump extruding several drugs from the neuron [13].
Temozolomide is a relatively new drug that has shown promise in treating malignant gliomas. Temozolomide has recently been approved in the USA for the treatment of adult patients with refractory anaplastic astrocytoma and in the European Union for treatment of GBM showing progression or recurrence after standard therapy. Concurrent radiation and Temozolomide increased median survival from 12 to 15 months, thus suggesting that Temozolomide acts as tumor-selective radiosensitizing drug [14].
Several trials are ongoing to search for more effective approaches. For example, the combination of radiotherapy, Lomustine and Temozolomide, yielded promising long-term survival data in patients with newly diagnosed glioblastoma, albeit with greater acute toxicity [15].
Antiangiogenic drugs represent another potential target for therapy of gliomas. High-grade gliomas demonstrate high levels of angiogenesis. Antiangiogenic drugs were developed to target vascular endothelial growth factor (VEGF) and VEGF receptor. Emerging literature suggests the efficacy of antiangiogenic therapy for recurrent high-grade gliomas. However, there is only partial response, and side effects may be important. Thus, further studies are required to define the population of patients with high-grade gliomas in whom this therapy is of benefit [16].
Radioimmunotherapy
Both systemic and locoregional radioimmunotherapy (RIT) have been proposed for targeting brain tumors. Monoclonal antibodies (MoAb) conjugated with high-energy β− emitters (131I, 90Y, or 177Lu) are used for this purpose. Stable binding of radiolabeled MoAbs to the tissue antigen is the basis for this approach. Tumor cells that do not express the antigen on their surface can be damaged by the “cross-fire effect” [17].
Systemic radioimmunotherapy did not achieve widespread use because of several factors that restrict the capability of large molecules such as antibodies to reach tissue antigens. These factors include the physiological BBB, areas of necrosis within the tumor mass, limited blood supply to the tumor, and inhomogeneous and inconstant antigen expression.
Therefore, locoregional administration is currently the preferred approach, although tumor-to-non-tumor uptake ratios may be suboptimal even with this procedure. Different strategies have been proposed to overcome this drawback and to improve the tumor/non-tumor uptake, such as the 3-step pre-targeting radioimmunotherapy (pRIT) approach based on the avidin–biotin system, which has been employed with some success [18]. A biotinylated, non-radiolabeled MoAb specific for the antigen of interest is injected (first step), allowing then a variable time to elapse (generally 2–3 days) for the non-tumor-bound MoAb molecules to clear from the circulation and from sites of nonspecific accumulation. The second step is injection of avidin, which binds with extremely high affinity to the biotin moiety of the biotinylated MoAb. Subsequently injected biotin labeled with 90Y (third step) binds avidly to avidin bound to the tumor-bound biotinylated MoAb (“sandwich” approach). Clinical trials used antibodies directed against tenascin, a tumor-associated glycoprotein of the extracellular matrix overexpressed in the stroma of GBM, but absent in the normal brain tissues. This approach has been used to treat patients with high-grade glioma in a nonrandomized, two-arm study. Disease-free survival in the treated patients (33.5 months) was significantly longer than in patients undergoing conventional therapy (8 months) [19]. Patients treated with pRIT and Temozolomide have a significantly longer disease-free survival compared to patients treated with pRIT alone (25 months vs. 17 months) [20].
Image Acquisition
PET Scanning
Patients are invited to fast for at least 6 h before tracer injection and to drink water. Between 220 and 370 MBq [18F]FDG is injected depending on type of acquisition (2-D vs. 3-D) and body weight. Preferred acquisition is 3-D, which has greater sensitivity and allows achieving better image quality. A 10–15 min static acquisition is performed about 45-min postinjection (uptake period). The patient is allowed to rest in a quiet dimly lit room with eyes closed in a condition of minimal audiosensory stimulation during the uptake phase, i.e., from tracer injection to start of PET scanning. Images are corrected for attenuation, scatter, and random coincidences. Subsequent image reconstruction is performed using filtered back projection or iterative reconstruction.
Using arterial input function, dynamic scanning, and kinetic analysis, it is possible to obtain a quantitative measurement of the parameter of interest, e.g., the glucose metabolic rate [21, 22]. Using noninvasive, i.e., without an arterial line approaches and a single static acquisition, it is possible to obtain qualitative or semiquantitative measurements. The standardized uptake value (SUV) and the tumor/non-tumor activity ratio are the most common semiquantitative indices.
Tracers other than [18F]FDG may have shorter uptake time, acquisition type, and data analysis approach, depending on the pharmacokinetics of the tracer.
SPECT protocols with blood flow tracers or amino acid tracers allow a single static acquisition, provide images of poorer spatial resolution, and do not allow absolute measurements.
PET/MR Coregistration
A critical issue of brain imaging is to identify anatomical regions that display pathological tracer uptake in the PET functional images. Accurate identification of morphological areas on PET images is currently felt unreliable. Thus, several algorithms have been developed to superimpose PET images on MR (or more rarely CT) images. Registration of PET to MR implies realignment and resampling of volumes to match the different pixel size of PET and MR images. Image reorientation is obtained through linear rotation across the x-, y-, and z-axes or by adding nonlinear warping. Traditionally, PET volumes are being resampled to the lower voxel size of MR that is used as reference image during the registration process. After successful registration, PET images can be superimposed onto MR. This process is referred to as image fusion. The two image modalities can be displayed along a continuum between 100% MR and 100% PET, thereby allowing to characterize and localize metabolic activity within the abnormal tissue. This approach is also useful for designing target volumes for external bean radiation therapy.
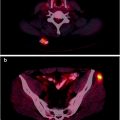
Irrespective of image registration, availability of MR images is always desirable for reading PET scans [23]. T1-weighted gadolinium-enhanced MR images are typically used for correlation with PET, while T2-weighted images can be used for evaluation of non-enhancing tumors and extent of peritumor edema.
Brain Tumor Imaging
Historical Perspective
Several tracers have been used for imaging brain tumors (Table 9.5) and mechanisms accounting for tracer uptake are multiple (Table 9.6). For several years, nuclear medicine techniques held a primary role for diagnosing brain tumors because CT entered into clinical practice only in 1973 and MR few years later. Pioneering studies for scanning brain tumors were carried out with 131I-labeled serum albumin and, subsequently, with 203Hg- or 197Hg-chlormerodrin by means of the rectilinear scanner developed by Benedict Cassen in 1950. Subsequently, with the advent of the scintillation camera (1959) [24], 99mTc-pertechnetate (99mTc), 99mTc-labeled radiopharmaceuticals, and 67Ga-citrate determined an increase in nuclear brain scanning that plateaued with the introduction of x-ray computed tomography. These radiopharmaceuticals display limited transport across the BBB in normal conditions and the rationale to their use stemmed from altered BBB permeability in CNS pathologies. Dynamic acquisition allowed studying perfusion of brain vessels referred to as nuclear cerebral angiography [25]. In presence of disease, all tracers displayed a good signal-to-contrast ratio, even though their use was nonspecific. 99mTc-DTPA and 99mTc-glucoheptonate, in addition to 67Ga-citrate, were thought to reflect some metabolic process by brain tumors. These radiotracers are nowadays abandoned due to overall poor spatial resolution of gamma camera and to the subsequent discovery of tracers with more specific binding processes.
Table 9.5
Radiotracers for brain tumor imaging
Biological process | Radiotracer |
|---|---|
BBB permeability | 99mTc |
BBB permeability and tumor metabolism | 99mTc-Glucoheptonate 99mTc-DTPA |
Multidrug resistance | 99mTc-Methoxyisobutylisonitrile 11C-verapamil |
BBB permeability and cellular vitality (ATP-dependent K+ channel transport) | 201Tl |
Cerebral blood flow | 123I-Iodoamphetamine (123I-IMP) 99mTc-Hexamethylpropyleneamine oxime (99mTc-HMPAO) 99mTc-Ethylcisteinate dimer (99mTc-ECD) [15O]water [15O]CO2 |
Blood volume | [15O]CO |
Cerebral oxygen metabolism | [15O]O2, [15O]CO2, and [15O]CO |
Brain pH | [11C]CO2 [11C]Dimethyloxazolidindione (11C-DMO) |
Glucose transport across BBB and metabolism | [18F]Fluorodeoxyglucose ([18F]FDG) |
Glucose transport across BBB | O-Methyl-11C-3-O-methyl-d-glucose |
Amino acid transport and protein synthesis | [11C]Leucine [11C]Methionine ([11C]MET) 123I-alpha-Methyltyrosine (123I-IMT) [11C]Tyrosine 18F-Fluoroethyltyrosine (18F-FET) alpha-11C-Methyltryptophane 18F-Proline |
Amino acid transport and dopamine metabolism | 18F-Fluoro-l-3,4-dihydroxyphenylalanine (18F-DOPA) |
Cellular proliferation | 18F-Fluorothymidine 18F-2-Fluoro-5-methyl-1-beta-d-arabinofuranosyluracil (18F-FMAU) |
Somatostatin receptor imaging | 111In-DTPA-octreotide
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|