Target volumes
Definition and description
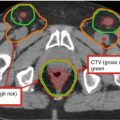
Breast
Clinical reference is required for breast tissue delineation. Breast tissue may be wired or borders may be placed clinically at the time of CT. Contour should include all glandular breast tissue. The cranial border should be below the head of the clavicle at the insertion of the second rib. Caudal border is defined by the loss of breast tissue. Medial border is at the edge of the sternum and should not cross midline. Lateral border is defined by the midaxillary line but is dependent on ptosis of the breast tissue. Anterior border is the skin or a few millimeters from the surface of the skin (for dose reporting) and the posterior border is the pectoralis muscles and muscles of the chest wall. The volume should not include these muscles or the ribs
Lumpectomy cavity
Seroma, surgical clips, and notable differences in the glandular breast tissue should be included. Comparison to the contralateral breast may be useful particularly when fluid and/or surgical clips are not present. All imaging studies should be reviewed prior to planning to assist in delineating this volume. This volume should not extend outside of the breast tissue
Lumpectomy CTVa
Lumpectomy cavity with a 1.0–1.5 cm expansion. This volume should not extend outside of the body or into the pectoralis muscles and/or muscles of the chest wall
Lumpectomy PTVa
Lumpectomy CTV with a margin based on setup uncertainty and predicted patient motion (generally 0.5–1.0 cm). This volume may extend outside of the patient surface and into the pectoralis muscles and/or muscles of the chest wall. Adjustments to this volume may be necessary for dose reporting purposes
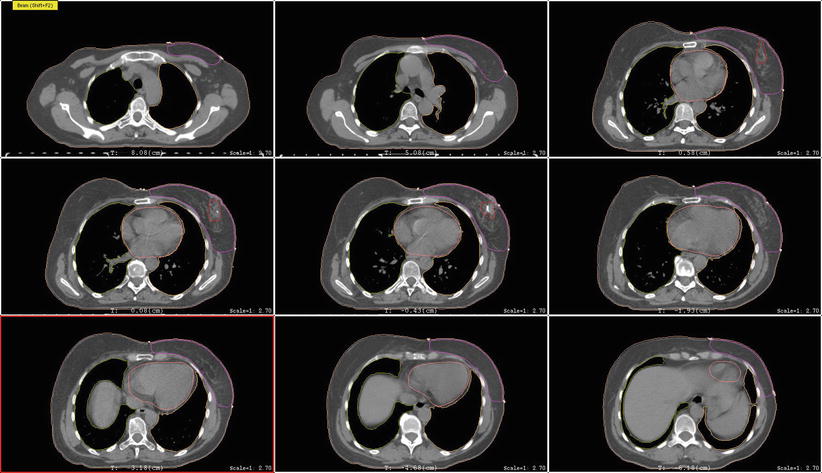
The breast tissue contour should include all visible glandular tissue as well as tissue encompassed in a wire (if used) or pendulous tissue. For some patients with tissue folds, it may be difficult to determine the lateral or superior edge of the breast tissues. In such cases, bony landmarks may be useful, but one should be careful to ensure all glandular tissue is included in the volume. The lumpectomy cavity should include all visible postsurgical changes and clips (if placed by surgeon). In cases where it is difficult to detect postsurgical changes, mammogram, ultrasound, and MRI should be utilized. Although breast cancers are not well visualized on CT, a preoperative CT may be helpful and should be reviewed if the patient had one completed. The heart should be contoured for all patients with left-sided breast cancer. Most physicians begin the heart OAR just below the pulmonary vessels. The left ventricle and LAD should be contoured if they can be identified (Fig. 1). This may be difficult on a noncontrast CT scan.
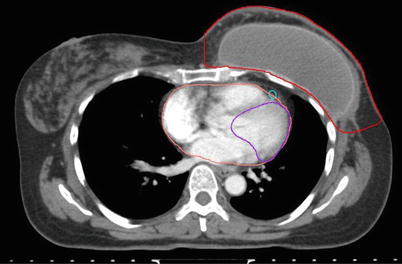

Fig. 1
Cardiac contours including the left ventricle, left anterior descending artery, and heart
5 Supine Positioning
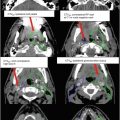
For supine positioning, the patient should be positioned on a breast board with arms above the head. We recommend neutral head position, and patient comfort should be ensured. CT images should be obtained to encompass entire breast volume with a generous margin. We recommend scanning from the chin to upper abdomen with a CT slice thickness of ≤3 mm. Some physicians prefer to place a wire around the breast tissue, while others may prefer to place a wire 2 cm under the breast tissue to assist with determination of the lower border. Most landmarks can be determined on CT scan and also seen on digitally reconstructed radiographs (DRR). The seroma can usually be identified on CT, but a wire on the incision may be helpful in determining seroma location. Caution should be taken, however, as incisions do not always correlate well with seromas and is often placed some distance away from the site of the breast cancer for cosmetic reasons. All glandular breast tissue and OARs should be contoured (Fig. 2). The physician or dosimetrist then determines tangent fields. These fields should encompass all breast tissue, collimated to be parallel with the chest wall. Gantry angles, collimation, and multileaf collimator (MLC) or cerrobend blocks can be used to best avoid heart and lung while treating the breast tissue and being careful to give an adequate margin for the seroma. After choosing and approving tangent fields, compensation for homogeneity is addressed. Some centers use wedges for compensation. Another option that may require more work on the part of the dosimetrist, but will provide better compensation and allow for more efficient delivery in the treatment room, is MLC field segment compensation. The concept is to reduce the dose in high-dose regions by adding an additional field or two that block the high-dose regions but do not reduce the dose coverage to the remainder of the breast and the resection site. MLCs are added to the field edges including any already determined blocking (i.e., heart block) and the energy of the beams should remain the same. To do this, each field is copied and named as a subfield. The dose is displayed in BEV windows and the high-dose areas noted and displayed alone (Fig. 3). This beam is then modified using the MLC leaf positions to cover this isodose volume. The number of monitor units for each of these fields is usually very small. Typically 2–4 subfields are necessary to reduce hot spots to less than 8 %. Another option is to use intensity-modulated radiation therapy (IMRT). IMRT uses inverse planning optimizing plans through a software system that takes constraints and desires for treatment plan and runs multiple iterations of treatment plans until the “best” treatment plan is achieved. This process is less labor intensive for dosimetry and results in a very homogeneous plan; however, it is more expensive.