Pelvic lymphnodes (%)
Para-aortic lymph nodes (%)
Low risk
<5
<5
Medium risk
5–10
<5
High risk
>10
>10
The most common sites of hematogenous spread are lungs, mediastinum, supraclavicular fossae, bones, and liver.
2 Diagnostic Workup Relevant for Target Volume Delineation
Patients with a diagnosis of endometrial cancer should have a thorough physical examination including pelvic exam as well as evaluation of the inguinal and supraclavicular lymph nodes.
During the pelvic exam, special attention should be given to evaluation of the vaginal vault, rectovaginal septum, and bilateral parametria and sidewalls. Exam under anesthesia is indicated if patient discomfort prohibits a thorough examination.
Patients often undergo transvaginal ultrasound for assessment of postmenopausal bleeding (Smith-Bindman et al. 1998).
Pelvic CT with oral and intravenous contrast could be considered for evaluation of the extent of the endometrial tumor.
On CT, endometrial carcinoma typically appears as a hypodense mass relative to the surrounding myometrium. It may appear as a diffuse, circumscribed, vegetative, or polypoidal mass within the uterine cavity.
If myometrial invasion is seen, it usually implies involvement of more than one-third to one-half of myometrial thickness.
Involvement of the cervix is visualized on CT as cervical enlargement >3.5 cm in diameter with heterogeneous low-attenuation areas within the fibromuscular stroma.
Parametrial or sidewall extension is seen by the loss of periureteral fat in the former and <3 mm of intervening fat between the soft tissue mass and the pelvic sidewall in the latter.
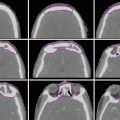
Dynamic contrast-enhanced magnetic resonance imaging (MRI) is the optimal method for detecting cervix invasion (Fig. 1) and myometrial invasion with an accuracy of 85–93 % (Frei et al. 2000).

Fig. 1
Magnetic resonance imaging (MRI) of endometrial cancer growing into the endocervical canal: (a) sagittal and (b) axial T2-weight sequences demonstrate thinning of the low signal intensity cervical stroma (white arrow) which suggests cervical stroma invasion (Image adapted from Imaging of Endometrial and Cervical Cancer by Patel et al. (2010))
The sensitivity of MRI in detecting lymph node metastasis is 27–66 % and the specificity is 73–94 % in surgically staged patients (Kitajima et al. 2011). However, PET is preferable if available. The sensitivity and specificity of PET for assessing regional lymph node metastases have been shown to range from 50 to 100 % and 87 % to 100, respectively (Kitajima et al. 2011).
3 Simulation and Daily Localization
Patients may be simulated in either a supine position or prone position. Prone positioning requires use of a belly board to allow setup reproducibility.
Endometrial cancer patients undergoing pelvic IMRT are typically simulated in the supine position. Immobilization with a customized cradle should be employed to minimize treatment setup error.
Patients should be simulated with a comfortably full bladder. At some centers, two scans are performed (full bladder and empty bladder) and the two scans are fused to generate an integrated target volume (ITV).
CT simulation should be done with ≤3 mm slice thickness. Intravenous contrast is helpful for blood vessel delineation.
In cases of vaginal involvement, a radiopaque marker should be placed at the caudal extent of the tumor.
Patients should undergo at least weekly imaging with megavoltage (MV) portal, kilovoltage (kV) imaging, or cone beam CT (CBCT) to verify treatment setup. Patients being treated with IMRT should undergo image guidance, preferably with daily imaging.
4 Target Volume Delineation and Treatment Planning
Suggested postoperative clinical target volumes (CTV) are detailed in Table 2 and are based on guidelines from Radiation Therapy Oncology Group (RTOG) and the Gynecologic IMRT Consortium (Small et al. 2008).
Table 2
Suggested target volumes used in endometrial cancer patients undergoing postoperative pelvic IMRT
Target volume
Definition and description
GTV
Visible disease on imaging and/or physical exam
CTV1
Vaginal cuff
Include any fat and soft tissue anterior and posterior to the vaginal cuff between the bladder and rectum
CTV2
Paravaginal/parametrial tissues, proximal vagina (excluding the cuff)
CTV3
Includes common iliac,a external, and internal iliac nodal regions
In patients with cervical stromal involvement, the presacral region is also included
The common iliac and external and internal iliac regions are defined by including the pelvic vessels plus a 7-mm expansion (excluding bone, muscle, and bowel) as well as all suspicious lymph nodes, lymphoceles, and pertinent surgical clips
Soft tissues between the internal and external iliac vessels along the pelvic sidewall are included
The presacral area consists of the soft tissues anterior (minimum 1.0 cm) to the S1–S2 vertebrae
Upper extent: 7 mm inferior to L4–5 interspace
Lower extent: superior aspect of femoral head (lower extent of external iliacs) and paravaginal tissues at level of vaginal cuff (lower extent of internal iliacs)
PTV1
CTV1 + 15 mm
PTV2
CTV2 + 10 mm
PTV3
CTV3 + 7 mm
The CTV is divided into three subregions: CTV1, CTV2, and CTV3.
In the intact/palliative patient CTV1 should include the entire uterus. CTV2 should include the paravaginal/parametrial tissues as in the postoperative setting plus 3 cm of the proximal vagina. CTV3 is the same as in the postoperative setting.
In patients with distal one-third vaginal involvement, the inguinal nodes should be contoured continuously from the external iliac nodes to 2 cm caudad to the saphenous/femoral junction.
If para-aortic nodes are involved, an extended field technique should be used by extending the cranial border of CTV3 superiorly to encompass involved nodes. The superior border should be chosen at the discretion of the treating physician.
Each CTV should be expanded differentially to form PTV1, PTV2, and PTV3 (Table 2). These three PTVs are then combined to form PTVsum.
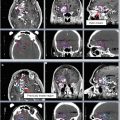
An additional boost of 5–15 Gy may be added for gross nodal disease or parametrial involvement at the discretion of the treating physician. This many be done sequentially or by an integrated boost (Figs. 2, 3, 4, and 5).

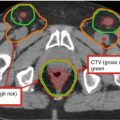
Fig. 2
A patient with stage IB grade 3 endometrial cancer who underwent a total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH BSO) with sampling of the pelvic lymph nodes. Pathology revealed deep (>1/2) myometrial invasion of a grade 3 tumor with extensive angiolymphatic invasion. Disease was limited to the fundus with no involvement of the lower uterine segment or cervix. Zero of 5 pelvic nodes were involved with tumor. She was treated with adjuvant intensity-modulated pelvic radiation therapy. Three clinical target volumes (CTV) are shown: CTV1 (green) consists of the vaginal cuff, CTV2 (blue) includes the paravaginal/parametrial tissues and proximal vagina (excluding the cuff), and CTV3 (red) consists of the common iliac, external, and internal iliac lymph nodes

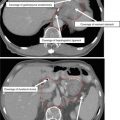
Fig. 3
The clinical target volume-3 (CTV3) (orange) is modified in endometrial cancer patients with cervical stromal invasion to include the presacral region

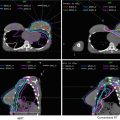
Fig. 4
Three separate planning target volumes (PTV) are generated in the postoperative endometrial cancer patient described in Fig. 2 (see Table 2). The final PTV used for treatment planning is generated by combining PTV1, PTV2, and PTV3. The resultant PTV (red) is shown in the figure encompassing CTV1 (green), CTV2, and CTV3 (yellow)