FIGO stage
I
II
III
Pelvic nodes (%)
15
30
50
Para-aortic nodes (%)
5
20
30
The most common sites of hematogenous spread are the lungs, mediastinum, supraclavicular fossae, bones, and liver.
2 Diagnostic Workup Relevant for Target Volume Delineation
Patients with a diagnosis of invasive cervical cancer should have a thorough physical examination including pelvic exam as well as evaluation of the inguinal and supraclavicular lymph nodes.
During the pelvic exam, special attention should be given to evaluation of the vaginal vault, rectovaginal septum, and bilateral parametria and sidewalls. Exam under anesthesia is indicated if patient discomfort prohibits a thorough examination.
Patients suspected to have urinary bladder or rectal involvement should undergo cystoscopy or rectosigmoidoscopy.
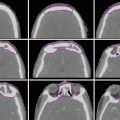
Magnetic resonance imaging (MRI) of the pelvis with intravenous contrast is helpful for determination of tumor extension into surrounding soft tissues and delineation of tumor during treatment planning (Fig. 1). MRI has been shown to be superior to CT and physical examination for determining size and extent of tumor invasion (Mitchell et al. 2006).

Fig. 1
Magnetic resonance imaging in a patient with a stage IVA cervical tumor with posterior bladder wall invasion (arrow) on axial contrast-enhanced T1 (a) and T2 (b) sequences
A computed tomography (CT) scan with contrast or positron-emission tomography (PET) scan is recommended for the evaluation of draining lymph nodes. PET scans are preferable if available, as they offer higher sensitivity and specificity than CT (Grigsby et al. 2001).
3 Simulation and Daily Localization
Patients may be set up in either a supine position or prone position for simulation. Prone positioning requires use of a belly board to allow setup reproducibility. Prone positioning allows the small bowel to fall out of the pelvis.
If intensity-modulated radiotherapy (IMRT) is going to be used, a supine position with a customized immobilization cradle should be employed to minimize treatment setup error.
CT simulation should be done with ≤3 mm slice thickness. Intravenous contrast is helpful for blood vessel delineation and should be used at the time of simulation unless medically contraindicated.
In cases of vaginal involvement, a radiopaque marker should be placed at the caudal extent of the tumor and consideration should be made for fiducial markers in the cervix.
The degree of bladder and rectal fullness should be made to duplicate that which is anticipated for daily treatment, i.e., if the patient is instructed to maintain a full bladder for treatment, she should be simulated as such. It is recommended to use a consistent bladder filling state (e.g., always full or always empty) for simulation and treatment. An enema may be applied at the discretion of the physician.
Patients should undergo at least weekly imaging with megavoltage portal, kilovoltage imaging, or cone beam CT (CBCT) to verify treatment setup. Patients being treated with IMRT should undergo image guidance with at least weekly CBCT.
4 Target Volume Delineation and Treatment Planning
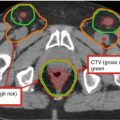
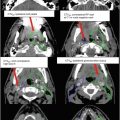
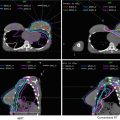
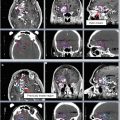
Suggested target volumes for the clinical target volume (CTV) based on guidelines from Radiation Therapy Oncology Group, the Gyn IMRT consortium, and the Japan Clinical Oncology Group (Lim et al. 2011; Small et al. 2008; Toita et al. 2011) are shown for both intact (Fig. 2) and postoperative (Fig. 3) cases.


Fig. 2
A patient with International Federation of Gynecology and Obstetrics (FIGO) stage IIIB (American Joint Committee on Cancer stage T3bN1M1) cervical carcinoma with para-aortic nodal metastases who underwent definitive extended-field intensity-modulated radiation therapy and concomitant cis-platinum. Three clinical target volumes (CTV) are shown: (CTV1) (blue), CTV2 (red), CTV3 (yellow) and boost volume (green) on a positron-emission tomography/computed tomography simulation. Please note that these are representative slices and not all slices are included. FDG fluorodeoxyglucose

Fig. 3
A patient with International Federation of Gynecology and Obstetric (FIGO) stage IB1 cervical cancer who underwent a radical hysterectomy and pelvic lymphadenectomy. Pathology revealed deep cervical stromal invasion as well as 3 of 15 positive nodes. She was treated with adjuvant intensity-modulated pelvic radiation therapy and concomitant cis-platinum. Three clinical target volumes (CTV) are shown: CTV1 (green), CTV2 (blue), and CTV3 (red)
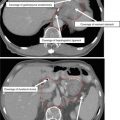
The CTV should be divided into three subregions: CTV1, CTV2, and CTV3.
CTV1 should include the gross tumor volume (GTV), cervix, and entire uterus for intact patients or 3 cm of the proximal vaginal cuff for postoperative patients.
CTV2 should extend 2 cm below the most inferior extent of vaginal disease.
CTV3 will include the common, external, and internal iliac and presacral lymph nodes with a 7 mm margin around the vessels and any additional visible lymph nodes, lymphoceles, or pertinent surgical clips.
CTV3 should not extend into adjacent bowel, bone, or muscle.
The upper border of the CTV3 should not extend above the aortic bifurcation and should begin no lower than the level of the inferior border of the L4–5 interspace.
The presacral nodes should be contoured until the superior border of the S3 vertebral body or the origin of the piriformis muscle is reached.
The external iliac nodes should be contoured until the external iliac vessels exit from the pelvis (approximated by the appearance of the femoral heads).
In cases with distal one-third vaginal involvement, the inguinal nodes will be contoured continuously from the external iliac nodes to 2 cm caudal to the saphenous/femoral junction.
If para-aortic nodes are involved, an “extended-field” technique should be used by extending the cranial border of CTV3 superiorly to encompass involved nodes. The superior border should be chosen at the discretion of the treating physician.
Each CTV should be expanded differentially to form PTV1, PTV2, and PTV3 (Table 2). The three PTVs will then be combined to form PTVsum.
Table 2
Suggested target volumes
Target volumes
Definitiona
Planning volumesb
Definitionc
CTV1
Gross tumor, cervix, and uterus or vaginal cuff.
PTV1
CTV1 + 15 mm
CTV2
Parametria and superior third to half of the vagina
PTV2
CTV2 + 10 mm
CTV3
Common, external, and internal iliac and presacral lymph nodes
PTV3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access