Cardiac and Extracardiac Tumors
Robert W. W. Biederman
The cardinal advantage that cardiovascular magnetic resonance (CMR) contributes to imaging of cardiac and paracardiac masses is surprisingly not its ability to visualize masses, but its ability to characterize tissue, potentially identifying the underlying histology and greatly enhancing the clinicians’ ability to distinguish between benign and malignant masses. This is accomplished by virtue of the T1 and T2 relaxation properties of tissue. The combination of high spatial resolution and the large field of view provide an unparalleled ability to depict surrounding structures, making CMR an ideal technique to evaluate masses in context. The modality provides a virtual surgeon’s view of masses and also serves as the pathologist’s microscope. It is therefore clear why an increasing number of surgical decisions regarding “go or no go” are being made largely based on CMR data.
Details of specific characteristics of each of the different types of masses have been well described elsewhere and accordingly this chapter will provide examples of how CMR adds to the clinical armamentarium, or provides the correct diagnosis when combined with information from other imaging modalities. Specifically, cases in which malignant diagnoses were suggested by another modality and were confirmed to be benign (or vice versa) will be presented.
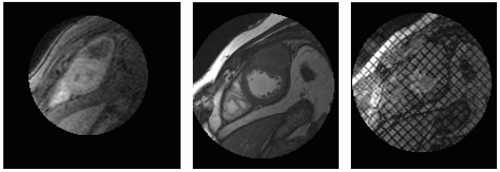
The general classification of cardiac tumors classically requires that a distinction be made between benign and malignant tumors. Certain key CMR features are very useful to differentiate malignant from benign tumors or paracardiac masses. Perhaps the most important observation is that most malignant tumors exhibit a signal that is isointense (or lower) with respect to the myocardium on T1-weighted images and high signal on T2-weighted sequences; whereas benign tumors generally exhibits isointense signal with respect to the myocardium in both T1– and T2-weighted sequences (see Fig. 13-1). In general, the tumor most commonly bright on both T1– and T2-weighted images is malignant melanoma (due to high melanin level), which is known to have a high predilection for metastasis to the heart (>60% likelihood). Pleural effusions ipsilaterally generally raise the suspicion that a tumor may be of malignant origin, especially if there is a concurrent pericardial effusion. Pericardial effusions in the setting of cardiac or paracardiac masses, counterintuitively, have only modest predictability for differentiating tumor etiology. The presence of infiltration is regarded as an ominous sign, portending malignancy. Malignancy is also indicated if the tumor is large (>5 cm) and bulky with central hypointensity on T1 weighting and a brighter central core on T2 weighting, suggesting a necrotic center, typically from the tumor outstripping its vascular supply and/or failure of neoangiogenesis. Heterogeneous tumor characteristics, except for myxoma or teratoma, strongly point towards malignancy. If the mass is heterogeneous, right sided, and there is a pericardial effusion, it is most often a malignant tumor (see Fig. 13-2) whereas if it is homogeneous, in the left heart, and there is no pericardial effusion, then the mass is almost always benign (see Figs. 13-3 to 13-7). Table 13-1 summarizes characteristics of several commonly encountered masses.
Contrast kinetics is very helpful in distinguishing malignant from benign masses. Contrast uptake is an ominous sign, indicating malignant potential. Most benign tumors do not have a well developed, nor needed, vascular supply. Myxomas may be an exception to this rule because they may have variable uptake with contrast administration. Radiofrequency (RF) tissue tagging is a technique that we have used extensively to help differentiate malignancy or “malignant” potential of benign tumors (see Figs. 13-1 and 13-8). The saturated bands of the tag excitation are applied in diastole across the myocardium and adjacent tumor. If there is no adherence or invasion, the tag lines should “slide” past each other during cardiac contraction, demonstrating freedom of attachment points. The pattern, when applied in orthogonal planes throughout the tumor/heart interface,
grants the imager a unique capability of assessing tumors, to predict before surgery whether a true surgical plane exists between heart and mass. This ability may be the second-most important feature that CMR renders to the oncologist and surgeon; the first being the ability to perform “virtual histology” on many tumors, saving the patient from the morbidity and mortality obligate in most diagnostic confirmatory strategies that include biopsy.
grants the imager a unique capability of assessing tumors, to predict before surgery whether a true surgical plane exists between heart and mass. This ability may be the second-most important feature that CMR renders to the oncologist and surgeon; the first being the ability to perform “virtual histology” on many tumors, saving the patient from the morbidity and mortality obligate in most diagnostic confirmatory strategies that include biopsy.
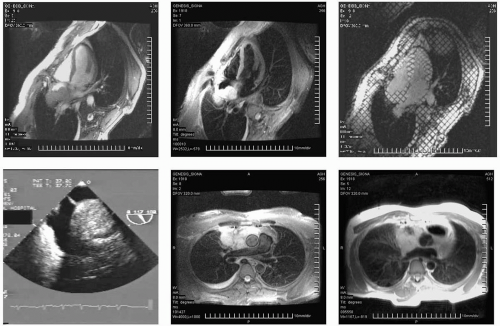 FIGURE 13-1 A collage illustrating the incorporation of several key sequences to interrogate location, structure and, most importantly, tissue characteristics of a retro-atrial (RA) mass. From top to bottom and left to right: triple inversion recovery (TIR), steady state free precession (SSFP), radiofrequency tissue tagging, a selected image (inner atrial septum) of the transesophageal echocardiogram (TEE), TIR demonstrating the superior extent of the mass, helping to absolutely exclude a myxoma, and a double inversion recovery sequence (DIR). Images relate to case study 1. |
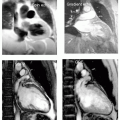
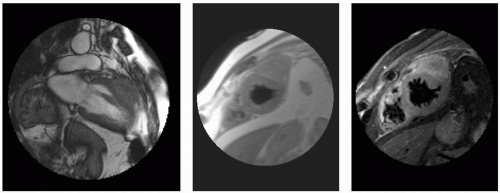 FIGURE 13-2 A paracardiac mass with high concern for invasion into the left ventricular (LV) myocardium. From top to bottom and left to right: two-chamber steady state free precession (SSFP), double inversion recovery (DIR), triple inversion recovery (TIR), perfusion, SSFP, and tagging. Images relate to case study 2. |
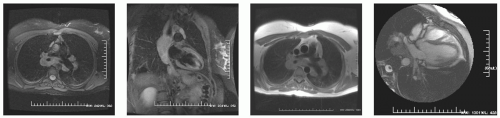 FIGURE 13-3 Sarcoidosis is demonstrated in these triple inversion recovery (TIR) sequences (left panels), double inversion recovery (DIR) (middle right), and steady state free precession (SSFP) (far right). Images relate to case study 3. |
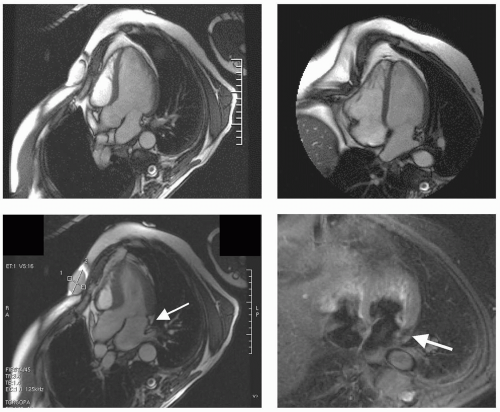 FIGURE 13-4 A series of steady state free precession (SSFP) sequences in the three- and four-chamber views with a triple inversion recovery (TIR) (lower right) demonstrating the mass to be that of an unusual intramyocardial lipoma (nulls on TIR). Images relate to case study 4. |
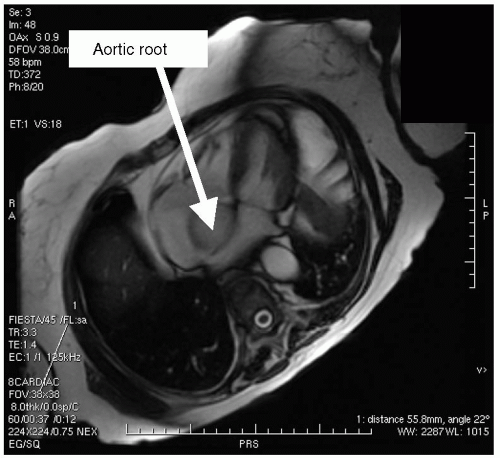 FIGURE 13-5 A steady state free precession (SSFP) selected four-chamber view that demonstrates a peculiar shadow of an apparent mass. This is a partial volume effect of what was seen on echocardiography as a mass and shown to be a torturous aortic root in an elderly patient. The blood flow and morphology on orthogonal imaging confirms this as a normal variant, not a mass. Images relate to case study 5. |
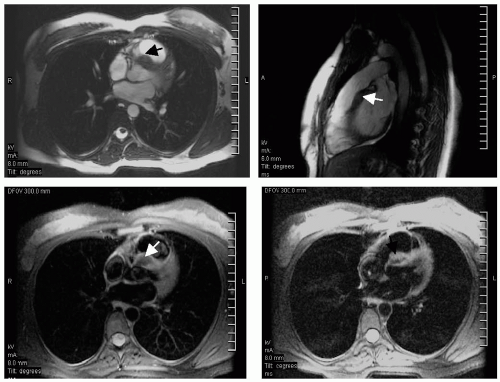 FIGURE 13-6 Steady state free precession (SSFP) images (top) and triple inversion recovery (TIR) sequences (bottom) depicting a benign dense myocardial fibroma (arrows). Images relate to case study 6. |
The increased capability to detect benign variances of normal is illustrated. The understanding of gross cardiac anatomy has been relatively stable over the last 100 years, reliant on well-established autopsy findings. The days of Rokitansky and Virchow seem to have heralded the end of significant cardiac anatomy discoveries. However, the advent of dynamic imaging by CMR and computed tomography (CT) provides a new window to view anatomic features. In a small study performed at our laboratory, the insertion of the papillary muscle (PM) into the apical left ventricular (LV) myocardium was seen in 401 of 401 patients (100%). In 392 out of 401 patients (97.8%), the appearance of the PM was not a uniform appearing muscle arising from the inner face of the LV endocardium, but was a fingerlike series of long, rootlike slender trabeculae carneae traversing >1 cm before inserting into the main body of PM, challenging our previous understanding of LV PM anatomy (see Fig. 13-9). We termed this finding the “cypress root.” Potential explanations accounting for the discord between autopsy and CMR include (a) the inability to see the fine detail by the naked eye during autopsy; (b) lack of belief that the PM was not a solid muscle; (c) even in live beating heart procedures such as open heart surgery, the true PM structure is not observable; and (d) myocardial systolic contracture at death (most often death occurs during systole not diastole). The capabilities of CMR to allow visualization of cardiac features in vivo afford
a useful tool to study living cardiac anatomy. Unlike the widely accepted belief that PMs uniformly arise from the LV floor, they resolve into a “cypress tree” rootlike structure with multiple thin projections before coalescing into a thick muscle head. Such observations have far reaching clinical implications in areas such as mitral regurgitation, post-myocardial infarction (MI) remodeling, and electrical transmission of the His-Purkinje system, while challenging standard teachings. Additionally, they clearly demonstrate the additive utility of high-resolution CMR imaging.
a useful tool to study living cardiac anatomy. Unlike the widely accepted belief that PMs uniformly arise from the LV floor, they resolve into a “cypress tree” rootlike structure with multiple thin projections before coalescing into a thick muscle head. Such observations have far reaching clinical implications in areas such as mitral regurgitation, post-myocardial infarction (MI) remodeling, and electrical transmission of the His-Purkinje system, while challenging standard teachings. Additionally, they clearly demonstrate the additive utility of high-resolution CMR imaging.
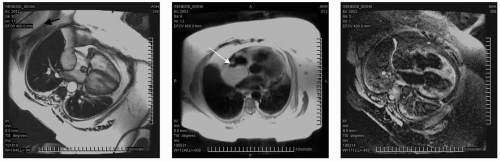 FIGURE 13-7 Steady state free precession (SSFP) images of a large space-occupying mass in the retro atrium (RA) (left and mid panel, double inversion recovery [DIR]) with triple inversion recovery (TIR) sequences confirming nulling of fat and a diagnosis of inner atrial septal hypertrophy, not a mass as she was suspected of having. Images relate to case study 7. |
The vast majority of cardiac masses are initially detected using transthoracic echocardiography (TTE), but typically cannot be unequivocally identified because of lack of differentiation of the echocardiographic signal. However, accurate mass identification is required before initiating therapy. In most centers, mass characterization is performed using a combination of transesophageal echocardiography (TEE), biopsy, catheterization, and CT scanning (see Figs. 13-10 to 13-19). CMR provides tissue contrast and permits flexible view selection sufficient for mass characterization. At our institution, from August 2002 to March 2005, CMR was performed using a gradient echo (GE) 1.5 T scanner in 76 patients referred for evaluation of cardiac masses detected by echocardiography. Contrast mechanisms exploiting intrinsic T1, T2, tissue relaxation properties, uptake kinetics of exogenous contrast agent, delayed hyperenhancement (DHE) and inversion contrast were used to identify fat and water signals to characterize masses. Of 76 patients studied, CMR was able to characterize 100% of masses into the following categories: 93.42% non-neoplastic, 6.57% malignant. Further breakdown was as follows: 38.15% (29 cases) as benign fat deposits, including lipomatous hypertrophy and epicardial fat; 5.26% were not masses but were normal variants, such as prominent crista terminalis (100% of these were referred as echogenic material with a suspicion of thrombus, fat, or malignancy); 6.57% were diagnosed as myxoma; 7.89% thrombus. Of those diagnosed by CMR as malignant, verification was obtained by histopathology, and 80% were shown to be malignant and 20% benign (see Figs. 13-20 to 13-23). Of masses defined as nonmalignant, the clinical sequelae were consistent with the CMR diagnosis. We concluded that in cases with echocardiographic suspicion of a cardiac mass, the wide range of contrast mechanisms (intrinsic and extrinsic) available to CMR permitted noninvasive characterization to be performed, differentiating with high specificity, non-neoplastic versus malignant lesions. These results suggest that CMR should be utilized following identification of cardiac masses to provide specificity and allowing the appropriate patient management.
TABLE 13-1 Characteristics of cardiac neoplasms | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||