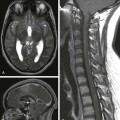
Chapter 36 Stroke is defined as a neurologic deficit persisting for more than 24 hours. Stroke may be caused by either cerebral ischemia or intracranial hemorrhage. The estimated annual incidence of stroke in children ranges from 2 to 13 per 100,000 person years and is among the top 10 causes of death in childhood.1 The presentation of stroke in children is sometimes heralded by the abrupt onset of a focal neurologic deficit, but often, especially in infants and young children, the symptoms are nonspecific and the diagnosis of stroke is frequently delayed. Although many children who have had a stroke recover completely, more than 75% will have a perceptible persistent neurologic deficit, and more than 40% will have major neurologic sequelae.2 Unlike adults, in whom hypertension, diabetes, smoking, and hypercholesterolemia are frequently identified as risk factors for the development of stroke, cerebrovascular arteriopathy, vascular anomalies, aneurysms, congenital heart disease, sickle cell disease, and hematologic abnormalities are among the most common predisposing conditions in children.1 More than half of all strokes in children are ischemic, caused either by an intrinsic vasculopathy or emboli from a remote source.2 Vascular malformations, aneurysms, and venous sinus thrombosis are the most common causes of hemorrhagic stroke. This chapter focuses on intrinsic vascular abnormalities as causes of ischemic and hemorrhagic pediatric stroke. Ischemic stroke in children has an annual incidence of between 2 and 3 per 100,000 children in the United States.3 The causes of ischemic stroke in children are diverse and include a variety of cerebrovascular entities in approximately 70% of children, such as arterial dissection, moyamoya disease or syndrome, sickle cell disease, isolated angiitis of the central nervous system or vasculitis, coagulopathy, or other known source; the remainder of cases are idiopathic in origin. More than one risk factor may be present. Twenty-five percent of children with ischemic stroke have coexistent cardiac disease.2 Metabolic disorders, such as mitochondrial diseases and hyperhomocysteinemia, should be considered in the differential diagnosis of ischemic stroke in children, especially if the distribution of the infarction does not conform to an arterial territory. The most common symptoms of ischemic stroke in children are hemiplegia, seizures, fever, dysphagia, headache, and altered level of consciousness. Headache and seizures are especially common in chronic ischemic conditions such as moyamoya disease.4 Imaging: The imaging features of ischemic stroke in children are variable and depend on the underlying cause. In acute arterial dissection, an end arterial distribution infarction is often present on computed tomography (CT) or magnetic resonance imaging (MRI). By contrast, in proximal, chronic steno-occlusive disease such as moyamoya disease (Fig. 36-1), infarctions may be absent, conform to an arterial territory, or lie within the border zones between the major vascular territories. In chronic multivessel steno-occlusive disease, there may be a shift in the location of the vascular border zones because of differential involvement of the various branches of the circle of Willis. Figure 36-1 A Noninvasive vascular imaging such as computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) typically shows an abrupt vascular cutoff with dissection and thromboembolic stroke. In moyamoya disease, stenosis or occlusion of the supraclinoid segment of the internal carotid and proximal middle or anterior cerebral artery is usually present, with numerous collateral vessels in the basal ganglia at certain stages of the disorder.5 Vasculitis causes irregular narrowing of medium or small vessels and is inconsistently demonstrated on CTA or MRA.6 Catheter angiography remains the gold standard for the imaging diagnosis of cerebrovascular disease in children and should be considered whenever a small vessel vasculitis is suspected or when surgical treatment is contemplated. Vascular anomalies are disorders of vascular development. Although congenital, they may only become symptomatic many months or years after birth. Intracranial vascular malformations are classified according to the vascular channels involved and the hemodynamics of the lesion (high-flow vs. low-flow).7 High-flow vascular anomalies occur when there is an abnormal connection between an artery and a vein that bypasses the normal arteriolar-capillary network. In arteriovenous fistula (AVF), the supplying artery communicates directly with the draining vein through a macroscopic fistula. In arteriovenous malformation (AVM), the supplying artery connects with the draining vein through a plexiform network of abnormal vessels, termed the nidus. Both AVF and AVM are further subdivided, on the basis of anatomic location, into dural, subarachnoid (vein of Galen malformation), pial, or parenchymal (Figs. 36-2, 36-3, and 36-5; e-Fig. 36-4).7,8 Figure 36-2 Five-month-old girl presenting with vomiting, lethargy, macrocephaly, and scalp vein distension with pulsating veins from dural arteriovenous fistula. Figure 36-3 A 12-month-old child presenting with macrocephaly and developmental delay. Magnetic resonance imaging (MRI) revealed a vein of Galen malformation. Figure 36-5 An 11-year-old girl presenting with severe headache, vomiting, and left leg weakness is found to have intraventricular hemorrhage and left frontal arteriovenous malformation. e-Figure 36-4 An 18-day-old child presenting with irritability, poor feeding, and vomiting is found to have a large left temporal hemorrhage on ultrasound. High-flow vascular malformations may produce symptoms and signs in several ways.7,9,10 The presence of an abnormal connection between a supplying artery and a draining vein allows for potentially rapid blood flow through the anomaly. In the absence of venous outflow obstruction, the malformation may cause high-output cardiac failure due to the lack of regulation of blood flow through the normal arteriolar and capillary network. The lower resistance through the malformation may result in diversion of blood away from the normal brain parenchyma (“steal” phenomenon), producing cerebral ischemia. High-flow malformations also expose the draining veins to arterial pressure, which may cause progressive stenosis of the vein, termed high-flow venopathy. High-flow venopathy may be a cause of seizures or cerebral atrophy due to impaired tissue perfusion from a diminished arterial-venous pressure gradient. Hydrocephalus can develop from increased venous pressures, that result in impaired resorption of cerebrospinal fluid (CSF) (see Fig. 36-3, A). Elevated venous pressure can also result in the development of a varix that predisposes to hemorrhage. Dural AVF or AVM comprise a meningeal arterial supply with dural venous sinus drainage and often present in the prenatal or immediate postnatal period as a cause of fetal or neonatal heart failure (see Fig. 36-2).7,10,11 The anomalies may include a single or multihole fistula or complex malformation. The abnormal connections are frequently located either in the vicinity or in the torcular herophili or involve dural venous sinuses that drain into the torcular herophili.
Cerebrovascular Disorders
Arteriopathy
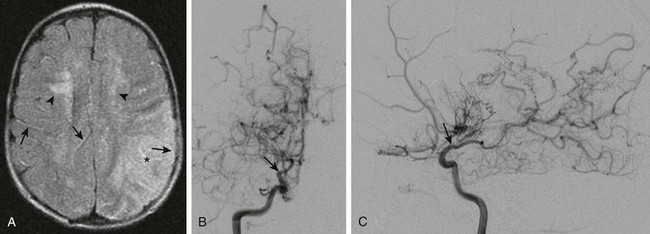
 -year-old child presenting with new-onset right-sided hemiplegia and imaging findings of moyamoya disease.
-year-old child presenting with new-onset right-sided hemiplegia and imaging findings of moyamoya disease.
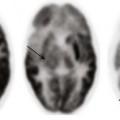
A, Axial fluid-attenuated inversion recovery magnetic resonance imaging scan shows a subacute infarct involving the left middle cerebral artery–posterior cerebral artery watershed territory (asterisk). There is periventricular white matter T2 prolongation due to chronic ischemic change (arrowheads). Linear sulcal signal abnormality is demonstrated bilaterally, consistent with slow flow (arrows) in leptomeningeal vessels. B, Frontal projection from digital subtraction angiogram shows severe steno-occlusive changes involving the distal right internal carotid artery (arrow), with occlusion of the middle and anterior cerebral arteries and multiple basal collaterals. C, Lateral projection again shows the distal tapering and occlusion of the supraclinoid internal carotid artery (arrow), multiple basal collaterals, and anterior shift of the posterior cerebral territory through pial collaterals to supply the posterior portions of the middle and anterior cerebral territories.
Vascular Anomalies
High-Flow Vascular Anomalies
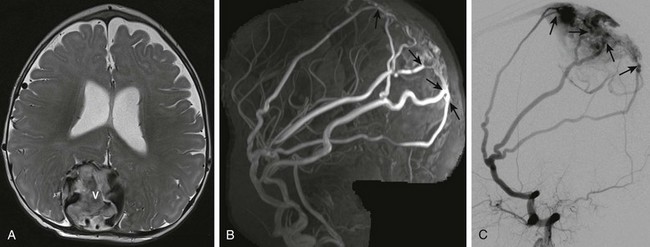
A, Axial T2-weighted image shows an enlarged, partially thrombosed superior sagittal sinus (V). Also noted are prominent middle meningeal arterial branches along the dural surface bilaterally. B, Maximum intensity projection reconstruction of the two-dimensional coronal magnetic resonance imaging venogram shows bilaterally enlarged middle meningeal arteries with fistulae (arrows) to the dilated, partially thrombosed superior sagittal sinus. C, Digital subtraction angiogram—lateral projection demonstrates the dural arteriovenous fistulae with the markedly enlarged middle meningeal artery branches directly communicating through several fistulae (arrows) with the superior sagittal sinus near the torcular herophili.
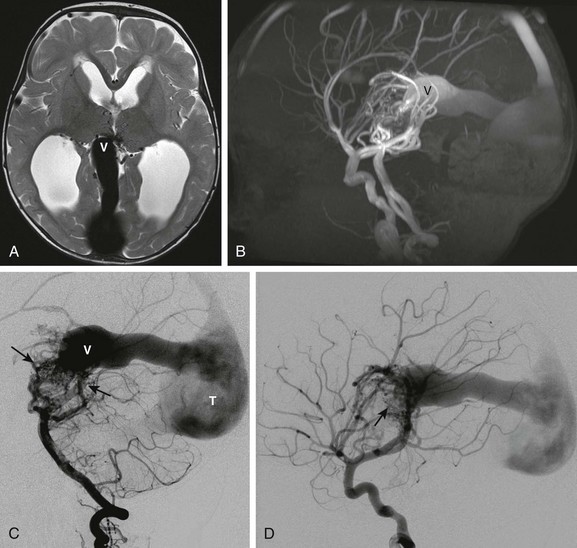
A, Axial T2-weighted image shows a dilated median vein of the prosencephalon (V). The straight sinus and torcular herophili are enlarged. Multiple small serpinginous signal voids are present medially between the thalami from enlarged branches from a primitive arterial arcade. Hydrocephalus is present from venous hypertension. B, Sagittal maximum intensity projection from an MRI angiogram confirms a high-flow arteriovenous connection with marked flow related enhancement in the varix and draining sinuses. C, Digital subtraction angiogram—lateral view of the left vertebral artery injection shows enlarged left vertebral, basilar, and posterior cerebral arteries supplying a primitive arterial arcade with a vascular nidus of abnormal vessels draining into the enlarged prosencephalic vein. D, Lateral view of the right internal carotid artery injection demonstrates additional anterior circulation supply to the malformation (arrow).
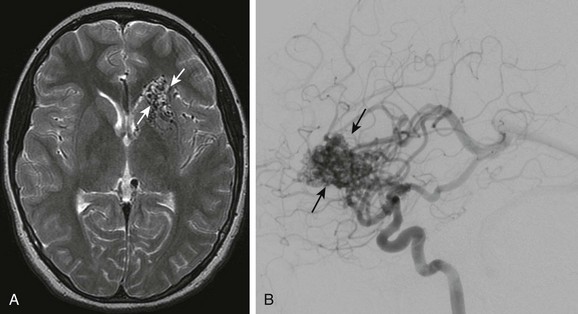
A, Axial T2-weighted image shows multiple signal voids in the left inferior frontal lobe involving the caudate head and basal ganglia. B, Lateral projection of a cerebral angiogram demonstrates the large vascular nidus (arrows) with early opacification of the internal cerebral veins and straight sinus.
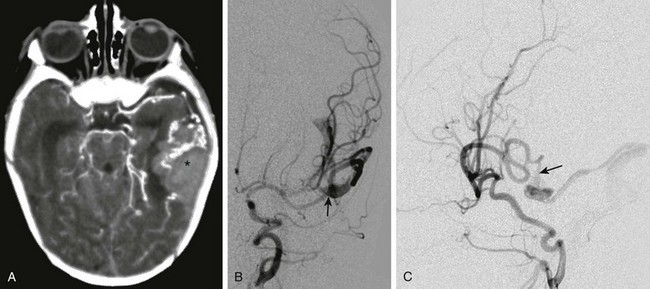
A, Computed tomography with intravenous contrast shows hemorrhage (asterisk) in the left temporal lobe associated with enlarged vessels. B, Digital subtraction angiogram—frontal projection shows a pial arteriovenous fistula (arrow) supplied by the left middle cerebral artery. C, The lateral projection from the angiogram demonstrates a fistula (arrow) between the middle cerebral artery and the dilated cortical vein.
Dural High-Flow Anomalies
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine