Shinichi Hori
BACKGROUND
Lung and Mediastinal Malignancy Treatment
Treatment of patients with advanced lung cancer or metastatic lung tumor, usually with serious symptoms such as respiratory distress, pain, or vascular stenosis of the superior vena cava or pulmonary artery, is often attempted. Options commonly taken are palliative radiotherapy or systemic chemotherapy. However, results suggest that these therapies give little benefit. In the case of palliative radiotherapy, the results are indeterminate and any improvement takes a long time to appear. Systemic chemotherapy is often applied despite a history of poor results and serious systemic side effects. Even if these therapies are at first effective, the new lesions created soon cause problems. It is evident that more effective treatment is needed, with fewer ensuing complications.
A new and promising treatment is selective chemoembolization of lung and mediastinal malignancy. Selective catheterization technique of the bronchial artery has shown by Neyazaki et al.1 in 1969: that blood supply to the primary lung cancer as well as to metastatic pulmonary malignancies has been provided mainly by the bronchial artery. This discovery has been followed up with the following study results. Preoperative induction chemotherapy using bronchial arterial infusion was reported to be effective.2 Bronchial artery infusion therapy combined with radiotherapy improved the prognosis of locally advanced lung cancer.3 Bronchial artery infusion for lung cancer was reported to be effective in a study with a small number of patients.4
In pathologic situations, the systemic arteries can penetrate into the lung and mediastinum and irrigate neoplastic lesions. Hemoptysis caused by bleeding from the bronchial artery, which shares systemic pressure, is well controlled by the bronchial artery embolization.5 In another study, the bronchial artery embolization for hemoptysis caused by lung cancer was effective to control bleeding with limited complications.6,7
The treatment of specific lesions in the thoracic cage, the mediastinum, and the lung field is now possible due to recent advances in diagnostic apparatus such as the three-dimensional (3-D) computed tomography (CT) scanner to digital angiography and to therapeutic techniques using microcatheters.
In this new approach, selective arterial infusion of antineoplastic agents concentrates the drugs in the target lesion and reduces systemic exposure.4 Embolization after the infusion helps retention of the drug by inhibiting flushing out by the arterial flow. Selective embolization using new spherical embolic materials does not cause the usual tissue damage because of reduced local irritability. Well-calibrated spherical material will allow controlling the occlusion level, which further avoids usual tissue damage. Arterial blockage may induce necrosis of tumor tissue in some cases. This selective chemoembolization of lung and mediastinal malignancy is a promising treatment procedure for patients who are suffering from various symptoms, such as severe cough or respiratory distress, caused by advanced cancer.
Local Breast Cancer Recurrence and Chest Wall Metastases
Difficult problems for patients are lesions in the chest wall or operated breast after the standard therapy for breast cancer. Patients may see the lesions and or identify them by touch. Usually, they cause intolerable pain, massive bleeding, and infection and seriously restrain the patient’s activity. They may invade the mediastinum or form nodular lesions in the lung. Sometimes, surgical resection is necessary to improve symptoms, but it is usually ineffective.8,9
A recent advance that has the potential to control tumor growth and active bleeding has been the efficient transarterial administration of drugs (anthracycline, cisplatin, docetaxel) with embolic material. Currently, greater precision is provided during the treatment of individual lesions due to relatively recent advances in angiography, such as superselective microcatheterization and calibrated microspheres.
DEVICES AND MATERIALS
Catheter System
A 4-Fr guiding catheter system is adequate for a transfemoral or transbrachial approach. A Cobra catheter (Terumo, Tokyo) is the first choice for femoral access with less risk of intimal dissection of the aortic wall or occlusion of the bronchial arteries. The Michelson’s shape or Shepherd hook type (Terumo, Tokyo) should be the second choice because they provide (higher) risk of intimal damage or temporary occlusion of the orifice of the bronchial artery. A coaxial microcatheter is mandatory for safe and selective catheter insertion. It is extremely important to avoid spasm or temporary occlusion of the bronchial artery. The maintenance of free antegrade flow through the blood vessels is key for effective embolization using flow-directed spherical embolic material.
The use of microcatheters is of particular importance when embolizing the right intercostobronchial trunk. Exclusive superselective catheterization of its bronchial branch will avoid potential occlusion of intercostal branches, which may supply the anterior spinal artery.10 The embolization of the anterior spinal artery is associated with paraplegia.
Antineoplastic Agent
Different kinds of antineoplastic agents are necessary. Necrotic effects or tumor shrinkage in the mediastinal or pulmonary tumor cannot be expected by embolization alone. The anticancer drugs and their combination are selected according the tumor types or patients’ treatment history or allergic reaction. Cisplatin, docetaxel, fluorouracil, anthracycline, or their combination is usually used for primary lung cancer. The total dose of antineoplastic agent can be reduced with selective tumor chemoembolization compared to systemic chemotherapy. We typically perform three sessions on average of chemoembolization.
Embolic Material
The spherical embolic material is the only option among the microparticles. Embolic materials that may cause proximal or permanent occlusion are not recommended. Polyvinyl alcohol (PVA) is not suitable because of the tendency of proximal occlusion. Glue is not suitable due to permanent occlusion. Liquid material such as Lipiodol11 is potentially dangerous for normal tissue (skin necrosis or damage). Metallic coils will close the arterial access and make it impossible for the sequential procedures, which are always necessary. HepaSphere (QuadraSphere; Merit Medical Systems, Inc., South Jordan, Utah), Embosphere (Merit Medical Systems, Inc., South Jordan, Utah), Embozene (CeloNova BioSciences, Inc., San Antonio, Texas), and Bead Block (Biocompatibles UK Ltd., Farnham, Surrey, United Kingdom) are spherical embolic materials. HepaSphere seems suitable because of its low irritability. The preferable size is 50 to 100 μm in dry state. HepaSphere is an expansible microsphere. The rate of expansion depends on the sodium concentration of the solution. When it contacts with physiologic saline and nonionic contrast material, it becomes four times and six times larger, respectively. The HepaSphere microsphere mixed with the solution with 10% of sodium chloride and nonionic contrast material (1:4) is two times larger. Five milliliters of this solution is put into the 25 mg of vial 10 minutes before use. Further expansion of microsphere up to four times can be expected in the arterial occlusion point. It results in the tight occlusion of the vessel.
Calibrated microspheres seem to improve the efficacy in arterial occlusion at the point of tumor vasculature. Drug-eluting microspheres do not have enough record regarding safety or efficacy. There are no reports to use drug-eluting microspheres for extrahepatic lesions. Our clinical experience is also limited regarding the drug-eluting HepaSphere for mediastinal and chest wall lesions. The role of embolic material is not only for the ischemic effect but also as modulator of chemotherapeutic agents, which can then remain longer in the target lesion than with simple infusion. We are planning to do an animal study to confirm hypothesis. However, it is common to obtain better clinical result from the combination use of antineoplastic agents and microspheres than the solo infusion of drugs.
ANATOMY
Mediastinal Circulation
The main artery for mediastinal circulation is the bronchial artery. There are many variations of bronchial arterial branching from the aorta.12 The details of the vascular anatomy are well described in the Chapter 18 (“Bronchial Artery Embolization” for hemoptysis management). Hilar lymph nodes and mediastinal tumors are generally supplied by this artery. Lung tumors are also fed by the bronchial artery.1 The right bronchial artery usually shares a common trunk with the right upper intercostal artery.
The small branches of internal thoracic arteries on both sides may feed the anterior part of the mediastinum. The small branches from the costocervical and thyrocervical arteries may also feed the upper part of the mediastinum. A direct tiny branch from brachiocephalic or subclavian arteries to the mediastinum should also be considered.
The intercostal artery is important if a tumor invades the chest wall. Careful attention to the spinal cord is essential to avoid spinal arterial occlusion, especially when performing embolization of intercostal arteries in the T8–L2 vertebral bodies segment.
The inferior phrenic artery sometimes penetrates the diaphragm and feeds the inferior part of the mediastinum.
Although a thoracic aortogram is recommended to evaluate the branching of the bronchial arteries,13 a 3-D analysis of dynamic CT with contrast is found to be more useful.
Most lung and mediastinal tumors, regardless of being primary or metastatic tumors, are seen as area of hypervascularity and neovascularity. Sometimes, the neoplastic lesion may show shunting of blood from the bronchial artery branches into pulmonary artery or pulmonary vein.14
Chest Wall Circulation
All branches from the subclavian artery, except for the vertebral artery, are involved with recurrent lesions in the chest wall, axillary nodes, and supraclavicular lymph node metastases. The intercostal arteries are potential feeders for chest wall lesions. Almost all chest neoplastic lesions, including lymph node metastases, are identified as hypervascular lesions compared to normal tissue.
TECHNIQUE
There is no specific angiographic technique for embolization of intrathoracic and chest wall malignancies. But compared to other organs, the diameter of their arterial feeders is generally smaller. Infusion and embolization should be done in state of free flow. For this reason, arterial spasm or intimal damage caused by catheterization should be avoided. Operator experience, careful manipulation of the arteries, and catheter insertion with a preshaped microcatheter without microguidewire may help decrease the incidence of complications. Other interventionalists prefer to carefully advance a microcatheter with a microguidewire coaxially through a 4-Fr to 5-Fr diagnostic catheter once the latter is in stable position at the aortic branch ostium (e.g., intercostobronchial or bronchial arteries).
CT examination during selective angiography is reliable for recognizing the anatomy of target arteries. Hybrid angiography suite–CT apparatus provides angiographic and CT examinations in the same room and at the same time, which helps our treatments from cost-effective standpoint. A cone-beam CT function of recent angiography suites may be helpful, but the diagnostic value is not good enough to evaluate tiny arterial supply. Multidirectional digital subtraction angiography (DSA) may also be useful to evaluate the arterial territory, but at the expenses of higher volume of contrast material and radiation dose.
Antineoplastic agents should slowly be infused to the target artery, avoiding backflow. A mixture with contrast material (1:1) is preferable to recognize the flow. Immediately after completion of infusion, embolization should be commenced. Embolic material should be mixed with contrast material to get good opacity to check the free flow state. The end point of embolization is not arterial occlusion but disappearance of tumor vasculature. Flow reduction observed on fluoroscopy during the injection of embolic material is a good sign that the embolization procedure is complete.
Bronchial artery embolization with drug infusion should be repeated (two or three times) to obtain a better local response and to promote a longer survival time.1
CLINICAL APPLICATIONS
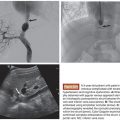
The main purpose of transarterial treatment for the lung and mediastinal tumors is the reduction of tumor burden. Airway stenosis due to compression or stenosis caused by tumor or direct invasion may cause serious symptoms. The pulmonary hilum lymph node metastases or tumor invasion may result in bronchial stenosis, which may consequently cause insufficiency of sputum expectoration. It may evoke obstructive pneumonia or atelectasis (Fig. 20.1). Irritation of bronchial mucosa causes severe cough or hemosputum. When the tumors compress the trachea, patients complain of respiratory distress or suffocation (Figs. 20.2 and 20.3). Mediastinal invasion or lymph node metastases often cause compression of superior vena cava or pulmonary arteries (see Fig. 20.2). They are always life-limiting factors as bronchial airway stenosis. Reduction of tumor burden by the embolization combined with antineoplastic agents can be obtained as long as superselective administration of drug and embolization can be performed.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree