Adverse Event Management
Targeted therapies such as sunitinib and everolimus have distinct typical adverse event profiles which differ to cytotoxic chemotherapies. The continual and sometimes prolonged nature of treatment with targeted therapies also leads to challenges in managing side effects. Raymond et al. [2] reported the most commonly experienced adverse events in patients with advanced pNETs receiving sunitinib; these were diarrhoea, nausea, asthenia, vomiting and fatigue (see Table 10.1). The majority of adverse events were grade 1 or 2 whilst the most common grade 3 and 4 adverse events were neutropenia (noted in 12 % of patients) and hypertension (noted in 10 % of patients). The adverse events which most commonly resulted in discontinuation of sunitinib were fatigue (4 % of patients), diarrhoea (2 %) and cardiac failure (2 %).
Table 10.1
Commonest side effects experienced in patients receiving sunitinib and everolimus in the phase III trials
Side effect | Sunitinib | Everolimus | ||
|---|---|---|---|---|
All grades (%) | Grades 3/4 (%) | All grades (%) | Grades 3/4 (%) | |
Diarrhoea | 59 | 5 | 34 | 3 |
Nausea | 45 | 1 | 20 | 2 |
Asthenia | 34 | 5 | 13 | 1 |
Vomiting | 34 | 0 | 15 | 0 |
Fatigue | 32 | 5 | 31 | 2 |
Stomatitis | 22 | 4 | 64 | 7 |
Rash | 18 | 0 | 49 | <1 |
Infections | Not known | Not known | 23 | 2 |
Treatment of patients with pNETs with everolimus also resulted in primarily grade 1 and 2 toxicities [3]. The commonest adverse events with everolimus were stomatitis, rash, diarrhoea and fatigue. The most common grade 3/4 toxicities were anaemia, hyperglycaemia, stomatitis, thrombocytopenia, diarrhoea, hypophosphatemia and neutropenia, all occurring with a frequency of 7 % or less. Adverse events led to discontinuation of treatment in 13 % of cases; the most common adverse events to result in discontinuation of treatment were pneumonitis, fatigue and interstitial lung disease (breakdown by event not provided). Everolimus in combination with octreotide LAR produced a similar pattern of adverse events with the most common adverse events being stomatitis, rash, fatigue and diarrhoea [4].
The trials examining the use of sunitinib and everolimus in NETs confirm that they are not without toxicity. The chronic nature of the treatment with targeted therapies also raises concerns about cumulative toxicity and potential late side effects. Assessment of patients with metastatic renal cell carcinoma (RCC) in an expanded access programme for sunitinib confirmed that with longer lengths of treatment (over 6 months) patients experienced a comparative increase in the incidence of adverse events compared to less than 6 months treatment (it must be noted that the schedule of administration differs between the two indications; 37.5 mg/day continuous daily dosing in pNET and 50 mg/day for 4 weeks followed by 2 weeks’ rest in RCC). There were, however, no new or unexpected toxicities seen and no increase in the rate of serious cardiac toxicity (grade 3 or greater) [6, 7]. This highlights the continual need to address emergent adverse event management with patients.
1.
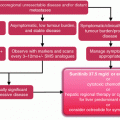
Prior to therapy: Adverse event management for pNET patients treated with targeted agents should begin prior to starting therapy. Careful history taking and examination is required to assess for co-morbidities, particularly those which overlap with the toxicity of the proposed treatment such as hypertension [8, 9]. Optimising a patient’s overall fitness is also important through for example addressing nutrition. It is also imperative to assess a patient’s psychological well-being and assess for conditions such as undiagnosed depression. An assessment of patients’ regular medication including complementary medicines should also be performed to rule out medications which could interact with the targeted medication, for example as sunitinib is predominantly metabolised by the cytochrome p450 (CYP), enzyme CY3A4 medications which affect CYP3A4 could influence sunitinib levels [10]; for example ketoconazole, an inhibitor of CYP3A4 doubled the area under the concentration–time curve (AUC) of sunitinib whilst rifampicin, an inducer of CYP3A4 reduced the AUC by half [11]. It is also important to screen for medications that may exacerbate potential side effects of the targeted agents such as QT prolongation. Pre-existing infections should be treated before a patient starts everolimus [12]. Everolimus has the potential to re-activate viral hepatitis infection; it is therefore necessary assess for risk factors of Hepatitis B and C and to undertake formal testing if at increased risk. Involving a multidisciplinary team in the patient’s management such as a podiatrist if there is pre-existing foot pathology prior to starting sunitinib may be helpful [8].
Routine blood tests should be performed along with specific bloods such as thyroid function tests for patients to be treated with sunitinib and glucose and lipid levels for patients to be treated with everolimus to rule out any undiagnosed conditions that may impact on a patient’s ability to tolerate treatment. It is then prudent to continue to intermittently monitor full blood counts, renal profile liver function tests and drug-specific test such as thyroid function [12, 13]. There is no standard routine for how often to check blood tests, but they are usually performed once in each 28-day treatment cycle provided the patient is stable with the exception of thyroid function tests which are often performed every 2 cycles [14].
2.
During therapy: It is also important that patients are educated appropriately about the side effects of the targeted agents they are to receive and how to tackle side effects [9, 15, 16]. It has been shown that patients may tolerate treatment better if they are prepared and can then tackle side effects prior to them affecting dose intensity [17]. It is also helpful if the patients have written information to back up what was discussed in the clinic. Illustrative aids may also be useful to aid patients recognising side effects such as PPE and everolimus induced rashes [15, 18]. Reinforcing the need for patients (or carers) to contact the specialist team promptly if they develop respiratory symptoms whilst receiving everolimus is also important. It may also be helpful to the patient if their family and friends are involved in the discussions about adverse event management to help them to manage adverse events outside the oncology clinic [15]. Frequent monitoring will be necessary initially, particularly during the first two cycles as patients are likely to require additional support and sources of advice such as contact with a specialist nurse to help them adjust to the new treatment and manage emerging adverse events [18]. In the initial cycle, it would be common to review the patient after 2 weeks of treatment and then review them at the start of each subsequent cycle.
Actively tackling adverse events as they occur is important to minimise their impact. It is important to distinguish between clinically relevant and less relevant side effects. Hypothyroidism, hair and skin depigmentation, neutropenia, PPE and diarrhoea seldom lead to treatment interruption or permanent dose reduction if appropriately managed. Fatigue and hypertension occasionally lead to treatment interruption or dose reduction. Pneumonitis, interstitial lung disease and cardiac failure are potentially life-threatening and may require treatment interruptions or discontinuation [2, 3]. It is important to consider techniques to manage adverse events whilst preserving dose intensity if possible. Strategies to tackle common adverse events experienced with everolimus and sunitinib will now be considered.
Adverse Event Management Strategies
Fatigue
Fatigue is a common adverse event associated with many targeted therapies and can be very disabling for patients significantly impacting on their quality of life. Fatigue is often multi-factorial with the patient’s cancer and co-morbidities potentially contributing to its severity in addition to the therapy itself. Identification and correction of reversible factors such as hypothyroidism and anaemia may improve fatigue. Fatigue can also be caused by underlying psychosocial problems or depression which should also be addressed if present. Dehydration can exacerbate fatigue so patients should be asked to maintain a good intake of fluids [18]. Counselling regarding adaptation of daily activities and routines according to energy levels may enable patients to cope with fatigue more effectively. Physical exercise may be beneficial for patients experiencing fatigue [15, 18] though some authors advocate daytime rest [9].
Diarrhoea
Diarrhoea is a common adverse event associated with both sunitinib and everolimus. Patient education again remains central to the management of diarrhoea [19]. Patients should be encouraged to keep diaries listing the severity and frequency of episodes of diarrhoea along with associated symptoms to aid physicians in managing it [20]. Recommendations about adapting diet include increasing foods that are high in fibre whilst avoiding foods noted to aggravate the diarrhoea such as spicy foods. The involvement of dieticians to enable patients to adapt their diets whilst maintaining good nutrition may be beneficial [18]. Dehydration associated with diarrhoea should be aggressively tackled through maintaining a high fluid intake and if necessary utilising rehydration solutions [8, 19]. Medication such as loperamide, diphenoxylate or opiates may be required to control diarrhoea. Patients should be given clear instructions about the most effective way in which to take these medications to control their symptoms and their adherence to this should be subsequently assessed.
Dermatological Adverse Events
Targeted agents can cause a range of dermatological adverse events including rashes, PPE, hair depigmentation, skin discoloration, pruritus and acute folliculitis. The causative mechanism for the frequent dermatological side effects is not fully known, but proposed mechanisms include microtrauma to capillaries leading to impaired repair, accumulation of breakdown products in keratinocytes and excretion of substances in sweat leading to high concentrations on certain areas of skin [21]. PPE, in which painful blisters and calluses occur in areas of friction and pressure, is a frequent adverse event associated with sunitinib. Patients should be advised to wear comfortable shoes with extra support from cotton socks and gel insole liners for cushioning. Patients may benefit from the involvement of podiatrists [15]. Application of topical alcohol-free emollients to the palms of the hands and soles of the feet after baths and overnight should be encouraged [18].
Rashes can be managed by encouraging regular moisturisation and the application of urea containing lotions to the skin. Long-term topical steroid use should be discouraged due to the risk of infection. Anti-histamines may help alleviate associated pruritus. Distinguishing a rash that is not clinically serious from a rash associated with hypersensitivity such as Stevens–Johnson syndrome involves assessment for bullous lesions, mucosal involvement and associated clinical symptoms such as elevated temperature [19].
Stomatitis
Stomatitis (usually oral) was noted as one of the commonest adverse events associated with everolimus therapy in trials with neuroendocrine patients. Patients require education about stomatitis and how to tackle it. Dietary modifications such as avoiding spicy, salty or acidic foods and eating foods which required less chewing may help [19]. Patients experiencing stomatitis may benefit from switching to paediatric toothpastes and using soft toothbrushes [8, 15]. They should be encouraged to use regular alcohol-free mouthwashes such as bicarbonate mouthwashes. They may experience symptomatic benefit from using mouthwashes containing paracetamol and using codeine and morphine if required to control pain. Topical anaesthetics and ice chips may also help alleviate the discomfort [15, 19].
Hypertension and Cardiovascular Adverse Events
Pre-existing hypertension should be corrected prior to commencing patients on sunitinib. Blood pressure should be regularly monitored either daily or three times a week [15] whilst a patient continues to receive sunitinib. If hypertension is subsequently diagnosed patients should be treated with appropriate anti-hypertensive according to their individual circumstances [19] and local/national guidelines. The optimal anti-hypertensive to use in patients receiving targeted agents is still an area of debate. Due to their renoprotective effects, the use of ACE inhibitors and AT-II receptor antagonists is recommended by some authors [22]. A number of anti-hypertensive agents such as verapamil and diltiazem use should carefully be considered in patients treated with sunitinib as they may affect metabolism by CYP3A4 [9]. Caution in the use of diuretics is also advised to the risk of developing diarrhoea and thus dehydration with targeted agents [22]. Lifestyle measures to help with hypertension such as regular exercise and a low-salt diet should also be recommended [8].
Cardiovascular adverse events such as congestive heart failure, left ventricular dysfunction, cardiomyopathy and prolongation of QT interval are associated with sunitinib with 2.4 % of patients with pNET developing cardiac failure in the phase III trial [2]. A thorough cardiovascular history and examination should be undertaken prior to commencing sunitinib [9]. Patients with myocardial infarctions, severe/unstable angina, symptomatic congestive heart failure, cerebrovascular accidents and transient ischaemic attacks within 12 months were all excluded from the phase III trial of sunitinib in patients with pNETs [2] and therefore would not be ideal candidates for treatment. Baseline electrocardiograms and if clinically indicated echocardiograms should be performed with regular electrocardiograms thereafter [18]. Patients should be educated about cardiovascular risks and the symptoms to monitor for. Oncologists also need to ensure they actively seek out symptoms or signs of cardiovascular compromise so that prompt investigation and treatment is undertaken if necessary.
Hypothyroidism
The rates of sunitinib induced thyroid dysfunction vary between studies up to 85 % in one retrospective review of patients with RCC [23]. In patients with pNET, 6 of the 86 patients randomised to treatment with sunitinib in the phase III study developed hypothyroidism [2]. The exact cause is unknown, but it is hypothesised to be due to prevention of appropriate binding of VEGF to thyroid cells and by impairing thyroid blood flow [23]. Prior to commencing treatment patients, thyroid function should be checked, and if any abnormalities are noted, these should be investigated and treated as appropriate. When patients are receiving sunitinib regular monitoring of thyroid function should be undertaken for example every other cycle [14]. Once detected, hypothyroidism should be managed as per standard medical practice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree