C. T. Sofocleous • P. Sideras • Elena N. Petre
Intra-arterial therapies directed to the liver take advantage of the dual hepatic blood supply and specifically the fact that liver tumors are fed by the hepatic artery, whereas the normal liver parenchyma gets its supply primarily from the portal vein.1 Surgery confers the best treatment option for colorectal liver metastases (CLM) but, unfortunately, only 20% of patients are eligible. Even more unfortunate is the 70% rate of patients developing new liver metastases after resection.1 There is, therefore, a tremendous need for nonsurgical therapies targeting liver metastases in patients with colorectal cancer. When using intra-arterial therapies in the setting of liver metastases, a drug (or radiation) injected in the hepatic artery will preferentially reach the tumor. Intra-arterial therapies to the liver include radioembolization or selective internal radiation therapy (SIRT), hepatic arterial chemotherapy (HAC), and transarterial chemoembolization (TACE) with oil or drug-eluting beads (DEB-TACE).2 Such treatments have been used as salvage therapies to manage progression after systemic therapies or other local or locoregional therapies.3–5 Intra-arterial therapies have also been used earlier in the course of the disease to achieve maximal response aiming to convert a candidate from nonsurgical to surgical. HAC with different chemotherapeutic agents allows 90% response rates and conversion to surgery up to 40% to 50% of initially inoperable patients.6 TACE and, in particular, DEB-TACE with irinotecan-loaded beads (DEBIRI) have been used for CLM with promising response rates and oncologic outcomes.7–9 Radioembolization or SIRT is a unique way of delivering a high tumoricidal dose of radiation to the tumor via the arterial pathway while minimizing toxicity to uninvolved liver. This therapy received original U.S. Food and Drug Administration approval when the use of resin microspheres in combination with HAC proved superior in terms of response and liver tumor control when compared to HAC alone.10 Subsequent results of SIRT were promising enough and led to further randomized control trials in the salvage setting as well as an ongoing trial with the use of SIRT with first-line chemotherapy (SIRFLOX).11–14 With these new techniques, as well as the much better established percutaneous thermal ablation, interventional oncology has emerged as an integral part of the colon cancer patient management in a multidisciplinary and tailored manner along with radiation, surgical, and medical oncology. Such tailored and multidisciplinary approach can achieve the best possible oncologic outcomes and specifically improve disease progression-free and patient overall survivals. In this chapter, we will discuss the indications for and the use of arterially directed liver therapies and we will review the literature with their respective expected outcomes.
INDICATIONS
Arterially directed liver therapies (ATs) are local or regional treatments that are most often used in patients suffering from colon cancer liver metastases without or with limited and controlled extrahepatic disease. Such therapies have been used as salvage treatments after failure of intravenous (IV) standard of care chemotherapy. Interestingly, the response rates were improved even in cases where the AT was administered with a drug that previously failed.12 Candidates for intra-arterial therapies are generally patients with a life expectancy of more than 3 months, ideally with Eastern Cooperative Oncology Group (ECOG) status of 2 or better and with a sufficient functional liver reserve. There is no uniform agreement on what is considered sufficient liver function, but a bilirubin level of greater than 3 mg/dL, an albumin level of less than 3 g/dL, and an international normalized ratio of 1.6 or higher are often deemed contraindications for any AT. Biliary obstruction should be corrected before any AT to prevent biliary necrosis and rapid deterioration of liver function, which can be fatal. Furthermore, patients with insufficient sphincter of Oddi are at increased risk of hepatic abscess formation, and in these cases, preprocedural and postprocedural biliary excreted antibiotic coverage is mandatory.15
Whenever such therapies are used as first-line treatments, also called induction treatments, their goal is to obtain the highest response as early as possible in the disease to convert a nonsurgical to a surgical candidate. Indeed, it has been shown that there is a linear correlation between the response rate and the resection rate when ATs were used as induction therapies.16 It is necessary to indicate that ATs are commonly used in patients who cannot undergo surgery or ablation. As such, most patients will have more than three tumors in the liver or larger tumors that cannot be resected or ablated with clear margins.17
HEPATIC ARTERIAL CHEMOTHERAPY
Intra-arterial hepatic chemotherapy has the goal of increasing drug concentrations in the metastatic deposits resulting in significant increase in response rates. The advantage of this route is proportional to the first-pass extraction of the drug by the liver and inversely proportional to body clearance. One drug that has been extensively used is floxuridine (FUDR), having a 95% liver extraction with liver exposures ranging from 100 to 300 times higher than with systemic administration.2 Also, the advantage of HAC applied with other chemotherapeutic agents was that exposure was several-fold increased by this method rather than IV perfusion alone. This allows intermittent administration with repeated course of chemotherapy over few weeks through the peripheral arterial access where a permanent subcutaneous port is linked to the intra-arterial catheter. This affords a better quality of life.
HAC requires placement of permanent intra-arterial catheter linked to a port usually placed surgically,1,18,19 although percutaneous placement is also feasible.20,21 During placement, the surgeon skeletonizes the hepatic arteries around the tip of the catheter that is placed in the gastroduodenal artery. This is done to prevent any extrahepatic delivery of the chemotherapeutic agent in the gastrointestinal tract. Often, the placement of the hepatic arterial infusion pump (HAIP) is performed while the patient is under general anesthesia for resection of the primary and/or a liver metastasis. At the same type, cholecystectomy is routinely performed.1 When no resection is anticipated, the HAIP can be placed through minimal invasive techniques, replacing largely the need for open surgery or repeated catheterizations.21 Similarly to the surgical ligation of arterial branches during intra-arterial hepatic pump placement, interventional radiologists ensure remodeling of flow before the insertion of the indwelling catheter so that there is only hepatopetal flow from the catheter’s tip where chemotherapy is released in a distribution that covers all the intrahepatic metastases, preventing the extrahepatic delivery of cytotoxic agents. First, any arterial branches that may cause delivery of the chemotherapy outside the liver should be occluded with coil percutaneously.22 Arteries that do not feed the liver such as right gastric and gastroduodenal are routinely coil-embolized to avoid gastrointestinal toxicity of extrahepatic drug perfusion resulting in gastroduodenal ulceration due to perfusion of chemotherapy in an extrahepatic vessel that remains patent beyond the tip.23 This can still occur when a small branch was not detected or recanalized after surgical ligation or coil embolization. A Tc-99m macroalbumin aggregate (MAA) study after pump placement is routinely performed to indicate that there is only hepatopetal flow without extrahepatic accumulation of tracer. In the event of extrahepatic accumulation of tracer, angiographic embolization may still be needed to correct the flow and salvage the intra-arterial pump/catheter for safe chemotherapy administration.24,25 Historically when combination of 5-fluorouracil (5-FU) and folinic acid was the standard regimen for IV chemotherapy in CLM, all clinical trials using 5-FU or FUDR demonstrated a better response rate for HAC than for IV treatments alone, with a few trials demonstrating survival benefit.26,27 In a more recent study, intra-arterial oxaliplatin combined with IV 5-FU demonstrated an overall response rate of 62% among the 39 assessable patients, including 17, 12, and 12 patients who had failed to respond to prior systemic chemotherapy with FOLFIRI, FOLFOX, or both, respectively.28 Of note, in the same report, an R0 surgical resection was performed afterward in 18% of initially nonresectable patients and ablation with clear margins (A0) in 2%. More combined chemotherapy schemes including a single intra-arterial agent such as FUDR plus two IV agents such as oxaliplatin and irinotecan allowed as high as 90% tumor response rates.29 In another study involving 36 patients with extensive nonresectable liver metastases (i.e., ≥4 metastases in 86% and bilobar disease in 91% of patients), HAC was used with oxaliplatin (100 mg/m2 in 2 hours) plus IV 5-FU and leucovorin (leucovorin 400 mg/m2 in 2 hours; 5-FU 400 mg/m2 bolus then 2,500 mg/m2 in 46 hours) and cetuximab (400 mg/m2 then 250 mg/m2/week or 500 mg/m2 every 2 weeks) as first-line treatment. Overall response rate (ORR) was 90% and disease control rate was 100%. Forty-eight percent of patients were downstaged enough to undergo an R0 resection and/or ablation.2
TRANSARTERIAL CHEMOEMBOLIZATION AND DRUG-ELUTING BEADS
There are several different techniques under the acronym TACE. The most common procedure is the intra-arterial injection of chemotherapy emulsified with Lipiodol Ultra-Fluide (LUF; Guerbet, Aulnay-sous-Bois, France) followed by injection of embolic material.30–34 Lipiodol was first injected into the hepatic arteries in the early 1980s because of its capacity to target and remain fixed in tumors; LUF was first used as a diagnostic tool for the evaluation of disseminated hepatocellular carcinoma30 and then emulsified with various drugs for the treatment of liver tumors.31 The emulsion produces oil drops in the arterial flow, and these drops have a propensity to go through the largest arteries (which are the tumor feeders) without entering the small ones due to the surfactant properties of the oil drops.32 With Lipiodol-TACE, ratio of drug concentration in the tumor compared to the healthy liver and to peripheral blood levels can be as high as 10 and 1,000 times, respectively.33 Embolization after chemo-Lipiodol increases the efficacy of treatment by prolonging contact of chemotherapy to the tumor cells and by adding ischemia to the highly hypervascularized tumor usually targeted with this treatment. Such embolization has been reported to induce failure of the transmembrane pump, thus increasing drug retention inside of cells.34
Regimens used to deliver TACE included (1) cisplatin, doxorubicin, mitomycin C, Ethiodol, and polyvinyl alcohol that had shown an ORR of 43% in one study35; median survivals of 33 months from initial diagnosis, 27 months from the time of liver metastases, and 9 months from the start of chemoembolization were documented, suggesting a possible improvement over reported survival time for systemic therapies alone35; and (2) mitomycin C alone (52.5%), mitomycin C with gemcitabine (33%), or mitomycin C and irinotecan (14.5%) showing an ORR of 63%.9
In 2006, the development of DEB loaded with irinotecan (DEBIRI) came for the first time in clinical practice for the management of CLM.36 DEBIRI loaded with irinotecan had a 75% reduced systemic plasma level compared with intra-arterial irinotecan alone.37
In a randomized study of two courses of DEBIRI (36 patients) compared with eight courses of IV irinotecan, 5-FU, and leucovorin (FOLFIRI; 38 patients) used to treat 74 patients who failed at least two lines of chemotherapy, the DEBIRI arm was met with statistically significant improvement of all oncologic outcomes including patient survival.38 Specifically, the response rates were 69% for the DEBIRI group compared with 30% for the systemic FOLFIRI group. Similarly, the 2-year overall survival (OS) was 56% compared with 32%, and the median OS was 22 months compared with 15 months for DEBIRI versus FOLFIRI groups.
Improvement in quality of life was of longer duration for the DEBIRI group (8 months) compared to the FOLFIRI group (3 months, P = .0002). Finally, overall cost was lower for the DEBIRI treatment arm.
In a multicenter, single-arm study of 55 patients who underwent DEBIRI after failing systemic chemotherapy, response rates were 66% at 6 months and 75% at 12 months, with an OS of 19 months and a progression-free survival of 11 months.5
A recent comparison study of DEBIRI versus radioembolization for salvage therapy for liver-dominant CLM including series of 36 patients reported similar survival for both treatments as salvage therapy, with median survival times of 7.7 months for the DEBIRI group and 6.9 months for the radioembolization (SIRT) group. The 1-, 2- and 5-year survival rates were 43%, 10%, and 0%, respectively, in the DEBIRI group and 34%, 18%, and 0%, respectively, in the SIRT group.4
Around 30% of TACE sessions are associated with adverse events during or after the treatment. The factors predictive of adverse events and significantly greater hospital length of stay are lack of pretreatment with hepatic arterial lidocaine (P = .005), more than three treatments (P = .05), achievement of complete stasis (P = .04), treatment with greater than 100 mg DEBIRI in one session (P = .03), and bilirubin greater than 2.0 µg/dL with more than 50% liver replaced by tumor (P = .05).39 Table 41.1 displays results of studies using TACE for the treatment of CLM.
RADIOEMBOLIZATION/SELECTIVE INTERNAL RADIATION THERAPY
Traditional external beam radiation therapy in patients with diffuse hepatic malignancy does not improve overall survival40 because liver tolerance for developing radiation-induced injury is low compared with the doses required for tumoricidal effect.41 The usual dose of normal liver tissue tolerance to radiation is 30 Gy, whereas the dose required to induce a tumoricidal effect of a solid tumor is 70 Gy or higher.42 These facts resulted in the idea of selective internal transarterial radiation with the delivery of radiotherapy yttrium 90 (Y90)–impregnated microspheres. Currently, two different Y90 microsphere products with a mean diameter of 20 to 35 µm are available: TheraSpheres (Nordion, Ottawa, Ontario, Canada), which are glass microspheres, and SIR-Spheres (SIRTeX Medical Limited, New South Wales, Australia), which are resin microspheres.
Radioembolization delivers targeted radiation therapy to unresectable liver metastases by the injection of β–emitting isotope Y90, which is bound to nondegradable microspheres into the arterial supply of the liver. These microspheres are unable to pass through the vasculature of the liver due to their relatively large size in comparison to the capillaries and are therefore trapped in the tumor capillaries.
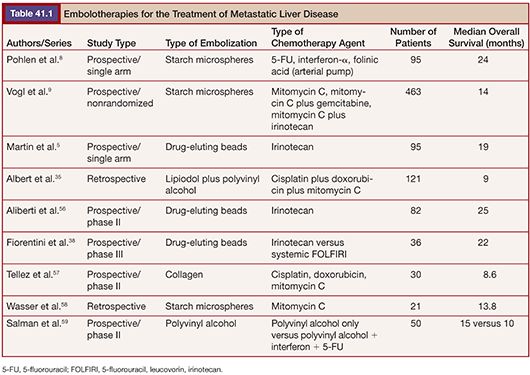
The physical properties of this radioactive isotope are a half-life of 64 hours and no γ-energy emission, thus allowing immediate release of the patient after treatment. The average range of radiation penetration in tissues is 2.5 mm to 1 cm. This allows delivery of high dose of ionizing radiation to the tumor with minimal radiation to surrounding tissue and thus causing considerably less toxicity to the normal liver.43 Criteria for radioembolization include patients with unresectable (and noneligible for ablation) CLM, patients with liver-only or liver-dominant disease, life expectancy of at least 3 months, and acceptable liver reserve. As a matter of fact, in a recent phase I trial, we demonstrated that SIRT can be safely used as a salvage therapy in heavily pretreated patients who progressed after multiple lines of systemic chemotherapy and HAC as well as resection (Fig. 41.1). Provided that the bilirubin level is low (≤1.5 mg/dL), the risk of radiation-induced liver failure is extremely low even in the most heavily pretreated population.44
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree