DRUG-INDUCED LUNG DISEASE
Drug-induced lung disease is often overlooked or misdiagnosed. The most common categories of drugs that result in lung disease include chemotherapeutic agents, cardiac medications, and antibiotics.
Drug-induced diffuse lung disease may be associated with a number of possible appearances on HRCT (Table 15.1). These appearances reflect the typical manifestations of different patterns of lung injury reviewed in other parts of this book and include many of the interstitial pneumonias reviewed in Chapter 9.
The most common patterns of lung injury associated with drug toxicity include pulmonary edema and pulmonary hemorrhage, diffuse alveolar damage (DAD), organizing pneumonia (OP), nonspecific interstitial pneumonia (NSIP), usual interstitial pneumonia (UIP), and eosinophilic pneumonia. Less common patterns of drug toxicity include hypersensitivity pneumonitis (HP), a sarcoid-like reaction, constrictive bronchiolitis, pulmonary vasculitis and pulmonary hypertension, desquamative interstitial pneumonia, and lymphoid interstitial pneumonia.
There are no HRCT findings that specifically suggest drug toxicity. Drug reactions must be considered in the differential diagnosis of the various patterns described above. A high degree of suspicion and correlation with medication history is necessary to make a confident diagnosis. The patterns of lung disease related to drugs are reviewed below.
Pulmonary Edema
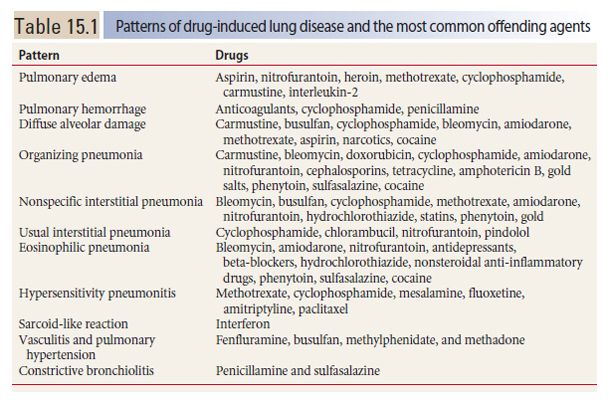
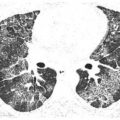
Hydrostatic pulmonary edema may result from drugs that affect the heart or systemic vasculature. An example is cocaine. HRCT findings are typical of any cause of hydrostatic edema (Fig. 15.1). Pleural effusion may be present.
Increased permeability pulmonary edema also may occur with drug treatment. Onset is usually sudden. HRCT findings are typical of pulmonary edema, including interlobular septal thickening, ground glass opacity, and to a lesser extent consolidation. This occurrence is typical of interleukin-2, but many other drugs are capable of causing increased permeability pulmonary edema. These include aspirin, nitrofurantoin, heroin, and cytotoxic agents such as methotrexate, cyclophosphamide, and carmustine. Unlike hydrostatic edema, pleural effusion is typically absent. Prompt resolution may occur with appropriate treatment.
Pulmonary Hemorrhage
Drug-related diffuse pulmonary hemorrhage is uncommon. Typical causes include anticoagulants, cyclophosphamide, and penicillamine. Hemoptysis may or may not be present. HRCT findings are typical of pulmonary hemorrhage, with bilateral patchy ground glass opacity or consolidation. Pleural effusion is typically absent.
Diffuse Alveolar Damage
DAD, the histologic pattern associated with acute respiratory distress syndrome (ARDS), is a common manifestation of drug toxicity. DAD is characterized by edema, intra-alveolar hyaline membranes, and acute interstitial inflammation, followed by fibroblast proliferation, and progressive fibrosis with collagen deposition.
Not all patients with DAD meet the clinical criteria for ARDS (see Chapter 8); this is particularly true in the setting of drug toxicity. The most common drugs associated with this pattern include carmustine, busulfan, cyclophosphamide, bleomycin, amiodarone, methotrexate, aspirin, narcotics, and cocaine.
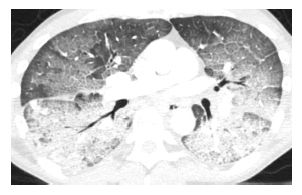
Figure 15.1
Pulmonary edema with cocaine use. Diffuse ground glass opacity and smooth interlobular septal thickening is present, representing the crazy paving pattern. This patient had an acute onset of pulmonary edema after the inhalational use of cocaine.
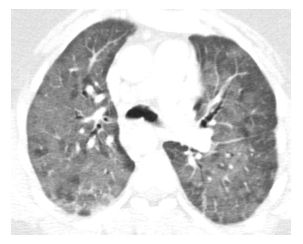
HRCT findings are identical to those of DAD from other causes. Diffuse ground glass opacity and consolidation are present (Fig. 15.2). Early, this may have a peripheral distribution, but the abnormalities quickly become diffuse. If early treatment is instituted, complete resolution of findings may be seen; otherwise patients may progress to ARDS. Eventually fibrosis may develop, often peripherally, and is anterior in distribution.
Organizing Pneumonia
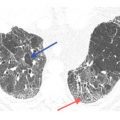
OP is a common pattern associated with drug toxicity. Conversely, drug toxicity is one of the most common causes of OP. This pattern is most commonly associated with carmustine, bleomycin, doxorubicin, cyclophosphamide, amiodarone, nitrofurantoin, cephalosporins, tetracycline, amphotericin B, gold salts, phenytoin, sulfasalazine, and cocaine.
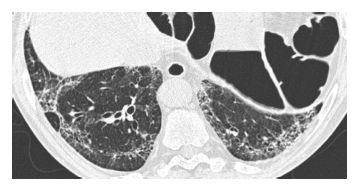
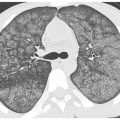
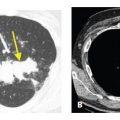
The predominant HRCT findings are the same as in other causes of OP, including bilateral patchy, nodular, or mass-like areas of peribronchovascular and subpleural consolidation (Fig. 15.3). The atoll sign or reversed halo sign, a peripheral rim of consolidation surrounding a central area of ground glass opacity (Fig. 15.4), is highly specific for OP. The findings of drug-related OP are indistinguishable from those of cryptogenic organizing pneumonia.
Figure 15.2
Diffuse alveolar damage due to carmustine treatment. Diffuse ground glass opacity is present in a patient being treated with carmustine for lymphoma. Pathologically, this corresponded to diffuse alveolar damage. This finding resolved after steroid treatment.
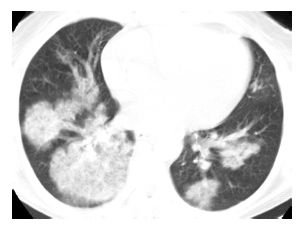
Figure 15.3
Organizing pneumonia with crack cocaine use. Patchy, bilateral, mass-like areas of consolidation are seen in a patient presenting with subacute symptoms after the recurrent usage of crack cocaine.
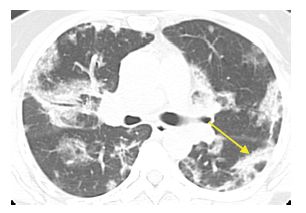
Figure 15.4
Organizing pneumonia with amiodarone. Patchy, bilateral, subpleural and peribronchovascular consolidation and ground glass opacity are present without evidence of fibrosis. Note the presence of the atoll sign in the left lower lobe (arrow). These findings are compatible with organizing pneumonia.
Nonspecific Interstitial Pneumonia
The most common cause of NSIP is connective tissue disease. Next on the list of likely causes is drug toxicity.
The most common drugs to cause this pattern include bleomycin, busulfan, cyclophosphamide, methotrexate, amiodarone, nitrofurantoin, hydrochlorothiazide, statins, phenytoin, and gold.
NSIP secondary to drugs may be cellular, fibrotic, or a combination of both. HRCT may be helpful in making this distinction; differentiation of cellular and fibrotic NSIP has significant implications for treatment, as cellular NSIP is most likely to respond to treatment and has a better prognosis.
Typical HRCT findings of NSIP include ground glass opacity and/or irregular reticulation with a subpleural and basilar distribution (Fig. 15.5). A peripheral distribution of abnormalities with sparing of the immediate subpleural lung is particularly suggestive of NSIP. The presence of ground glass opacity, with or without reticulation, suggests cellular NSIP. Reticulation associated with traction bronchiectasis suggests the fibrotic subtype of NSIP. Honeycombing is typically absent or minimal in extent, but when present indicates fibrotic NSIP.
Figure 15.5
Nonspecific interstitial pneumonia with amiodarone. Peripheral and basilar irregular reticulation and mild traction bronchiectasis are seen. There is subpleural sparing. After cessation of the drug and treatment with steroids, the majority of these abnormalities resolved.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree