, Stephen T. Haley2, Tara Bullard1, Jeffrey Davis1, Thomas K. Borg1 and Louis Terracio3
(1)
Department of Cell and Developmental Biology and Anatomy, School of Medicine, University of South Carolina, Columbia, SC, USA
(2)
Beckman Coulter Translational Solutions Business Center, San Diego, CA, USA
(3)
College of Dentistry, New York University, New York, NY, USA
Abstract
Detailed methods are provided for the preparation and confocal imaging of cardiac myocyte development and differentiation. Examples include protocols for the analysis of cultured myocytes as well as vibratome sections of hearts from embryonic and adult tissue. Techniques include routine labeling of F-actin with phalloidin as well as multiple labeling protocols for colocalization studies and cell volume analysis.
Key words
Cardiac myocyteCardiovascularConfocal microscopyImage analysis1 Introduction
The heart is a dynamic tissue comprised of a number of cell types that are continually remodeled in response to a variety of mechanical and chemical factors during development, normal cardiac contraction, and disease. The majority of the heart is comprised of two primary cell types, myocytes and fibroblasts, and others such as endothelial and smooth muscle cells that are associated with the coronary vasculature. Cardiac myocytes represent the bulk of the heart mass, and the majority of this chapter will present techniques we have used to image these cells by confocal microscopy.
The high-resolution, high-contrast, and three-dimensional imaging capabilities of confocal microscopy provide an exceptional technique to investigate the changes that occur in the structure of myocytes during developmental and pathological processes. In the following pages, we will present several protocols that we have used to examine how cultured and in vivo cardiac myocytes respond to the variety of signaling events to which they are continually exposed. For the most part, the techniques presented could also be used to image any of the cell types present in the heart by modifying the probes used.
1.1 Phalloidin Staining
Before detailing specific protocols, it is appropriate to discuss phalloidin staining and its role in the study of cardiac myocytes. Phalloidin is from the poisonous mushroom (Amanita) and binds specifically to filamentous or F-actin, which is a primary component of the contractile apparatus of cardiac myocytes. By conjugating a fluorochrome to phalloidin, it is possible to obtain a highly specific and clean stain that can provide information about the architecture of the myocytes. At the concentrations commonly used for staining (typically a 1:20 up to a 1:100 dilution of purchased stock or 0.33–0.066 μM dilution), the fluorescence obtained from phalloidin is linearly related to F-actin concentrations with little or no binding to non-sarcomeric or G-actin making it suitable for quantitation of F-actin [1, 2].
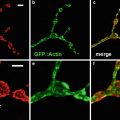
Figure 1 shows a generalized diagram of cardiac myocyte structure and the location of the F-actin thin filaments in the sarcomeres. Note that in the region of the H band, there is no F-actin present. If myocytes are stained with a fluorochrome-conjugated phalloidin, these areas will be unstained while areas with F-actin will be stained. This staining pattern results in the characteristic banding pattern observed when cardiac myocytes are stained with phalloidin. When not limited by the number of available channels, it is our practice to routinely perform a phalloidin stain to outline the structure of the myocytes. We then use the other available channels for staining of other cell types and proteins associated with the myocytes (Fig. 2).



Fig. 1
Diagram of a cardiac myocyte showing many of the major structural features that are commonly examined by confocal microscopy. When stained with a fluorochrome conjugated to phalloidin, the areas with F-actin thin filaments are labeled. The H bands, which lack F-actin, remain unstained resulting in the characteristic striated appearance of myocytes stained with phalloidin

Fig. 2
A single optical section through a vibratome section of an adult mouse heart that has been triple labeled for F-actin with Alexa-488 phalloidin (green), connexin 43 which is a component of intercalated disks (red ), and discoidin domain receptor 2 (blue) which is a stain for fibroblasts
1.2 Cardiac Myocyte Structure
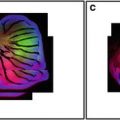
Early in mammalian development, the heart is a straight tube that undergoes a complex looping process that results in the four-chambered adult heart. During this looping process and subsequent maturation of the heart, cardiac myocytes are exposed to significant changes in mechanical force related to myocyte contraction and an increase in blood pressure [3]. In addition a number of chemical mediators, such as growth factors and hormones, affect cell division and differentiation during this time frame [4, 5]. These various stimuli induce significant changes in myocyte phenotype that results in initially round, undifferentiated cells becoming spindle shaped with a well-developed and architecturally complex structure (Fig. 3a, b).


Fig. 3
Single optical sections of an embryonic day 13.5 mouse heart (a), the left ventricle of a normal adult heart (b), and the left ventricle of a hypertrophied heart entering the transition to heart failure (c). All sections were stained with rhodamine phalloidin. Arrows in (a) indicate developing myofibrils on the edges of rounded myocytes. The arrow in (b) indicates an intercalated disk from a normal adult myocyte. The arrow in (c) indicates branching of myofibrils associated with severe cardiac hypertrophy and changes in the distribution and organization of intercalated disks
Following birth the normal beating of the adult heart and flow of blood past the cells of the heart also exposes the myocytes to a constantly changing environment to which they often respond by structural remodeling. In addition to changes associated with the normal development and function of the heart, a number of pathologies also result in phenotypic changes in myocyte structure and their surrounding extracellular matrix [6–8]. Many of these pathological changes result in myocyte hypertrophy and disruption of the architecture of the contractile components of the cell that may lead to severe cardiac dysfunction and death. Although transmission electron microscopy is necessary to image many of the minute structural changes that occur, others are readily imaged by confocal microscopy (Fig. 3c).
2 Materials
The materials and methods we use are dependent upon whether cultured myocytes, whole embryos, or sections of heart are being processed. Protocols for each of these types of sample will be presented. Unless otherwise stated, reagents can be purchased from Sigma (St. Louis, MO). All images were collected with a BioRad MRC1024ES confocal microscope equipped with an argon/krypton laser on a Nikon E800 platform.
2.1 Standard Buffers, Fixatives, Fluorochromes, and Mounting Media
2.
Phosphate buffered saline (PBS):
1,000 ml deionized water.
7.2 g NaCl.
1.43 g Na2HPO4.
0.43 g KH2PO4.
0.02 % sodium azide may also be added to prevent bacterial growth.
A 5× stock solution can also be prepared by using 200 ml of water rather than 1,000 ml.
25 mM KCl.
3.
Fixatives: Our fixative of choice is 2–4 % paraformaldehyde prepared in PBS at a pH of 7.2–7.4. To prepare 100 ml of paraformaldehyde, add 4 g of paraformaldehyde powder to 100 ml of PBS and gradually heat to 60 °C under a fume hood. To clear the solution, slowly add drops of 1 N NaOH while stirring. Do not allow the temperature of the solution to exceed 65 °C. The final pH can be adjusted with NaOH or HCl if necessary.
If antibody efficiency is not optimum with aldehyde fixation, an alternative fixative for cell culture protocols is methanol used at 4 °C. However, phalloidin staining is poor when methanol fixation is used.
4.
Washing buffer: 0.1 M glycine in PBS to block free aldehyde groups. If intracellular components are being stained, 0.1 % Triton X-100 may also be added to permeabilize cell membranes.
5.
Blocking buffer: 1 % BSA (IgG- and protease-free) in PBS to block nonspecific staining. If background problems exist, 5 % normal serum from the host of the secondary antibody may be added to the blocking buffer. For example, if the secondary antibody was made in a donkey, use 5 % normal donkey serum as an additional blocking agent.
6.
Phalloidin conjugated to fluorochrome of choice (Molecular Probes, Eugene, OR).
7.
Primary and fluorochrome-conjugated secondary antibodies diluted to desired concentrations in PBS-1% BSA blocking buffer. Antibodies should be centrifuged at 4 °C to remove particulate matter.
8.
Mounting media: 2.5 g DABCO (1,4-diazabicyclo[2.2.2]octane) dissolved in 25 ml of PBS and mixed with 75 ml of glycerine. Store at 4 °C. DABCO/PBS/glycerine remains fluid and specimens mounted with this mixture are routinely sealed with fingernail polish and stored in the dark at 4 °C. Gloves must be used when handling DABCO solutions (see Notes 2 and 3).
2.2 Additional Solutions for Processing Cultured Cardiac Myocytes
1.
Nifedipine (see Note 4): Nifedipine should be made fresh for each use. Mix 52 mg of nifedipine in 10 ml of 70 % ethanol, vortex, and use at a concentration of 1 μl per ml of cell culture media.
2.
CMFDA: To determine changes in cell volume, myocytes can be loaded with Cell Tracker™ Green 5-chloromethylfluorescein diacetate (Molecular Probes, Eugene, Oregon) diluted in DMSO to produce a 20 mM stock solution. Stock can be stored at −20 °C.
2.3 Additional Solutions for Processing Embryonic Hearts
Solutions and protocols for processing embryonic hearts depend on the age and size of the embryos. For our purposes, rat embryos less than embryonic day 9.5 and mouse embryos less than embryonic day 10.5 are processed intact. After these time points, embryos are too large and the tissue too dense for imaging through the entire heart. To image internal structures such as developing ventricular trabeculae and cardiac valves of older embryos we typically vibratome section the embryo. If three-dimensional reconstructions are necessary, the added thickness of vibratome sections is often preferred over cryo- or paraffin-embedded sections (see Note 5). For embryos between embryonic days 10–15, entire embryos are embedded in 13 % acrylamide to provide support for the embryo while sectioning. For older embryos, the thorax region is isolated and embedded in acrylamide.
Imaging of Entire Embryo Without Sectioning
Bovine testicular hyaluronidase—1,500 units/ml.
Imaging of Embryos with Vibratome Sectioning
30 % acrylamide stock (mix 30 g electrophoresis grade acrylamide and 0.8 g bisacrylamide cross-linker in 100 ml distilled water).
PBS.
10 % tetramethylethylenediamine (TEMED) stock.
2 % ammonium persulfate.
Tissue-embedding molds.
3 Methods
The following methods have been selected because they are representative of the techniques used in our laboratory. Some variation in the basic techniques does exist dependent upon the antibodies used. However, the protocols presented can serve as starting points for additional studies. Procedures for the culture of cardiac myocytes [9, 10] and whole embryo culture [2–4] have been published elsewhere and will not be covered here.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree