(1)
Department of Anatomy and Developmental Biology, Monash University, Clayton, VIC, Australia
(2)
Centre de Morphologie Mathématique, Ecole des Mines de Paris, Paris, France
(3)
Department of Genetics and Development, Columbia University, New York, NY, USA
Abstract
The structure of the ureteric tree in developing mouse and rat kidneys has previously been quantified in two dimensions. While this type of analysis may provide evidence of changes in ureteric growth, these measurements are effectively inaccurate, as the ureteric tree is a three-dimensional (3D) object. Here we describe a method for measuring the ureteric tree in three dimensions. This technique involves (1) culture of the metanephric kidney at embryonic day 12 (mouse) or 14 (rat), (2) whole-mount immunofluorescence to selectively stain ureteric tree epithelium, (3) confocal microscopy to obtain a complete Z series through the ureteric tree, and (4) image analysis algorithms to binarize, skeletonize, and measure individual branch lengths in 3D. This method has been extended to analysis of the same ureteric tree over time (4D). The results obtained provide accurate and precise quantitation of ureteric tree growth in the developing mouse or rat kidney.
Key words
KidneyConfocalMetanephrosDevelopmentUreteric treeThree-dimensionalMeasurementImage processing1 Introduction
The mammalian kidney (metanephros) develops through a series of inductive molecular interactions between the epithelial ureteric bud and the metanephric mesenchyme. Branching morphogenesis of the ureteric bud results in the formation of the ureteric tree and ultimately the collecting duct system. Simultaneously, the tips of the ureteric tree induce the formation of nephrons, the functional units of the kidney. Since only the tips of the tree have the capability of nephron induction, the efficiency of ureteric branching is a key regulator of nephron endowment in the adult kidney. Moreover, the pattern of ureteric branching morphogenesis is thought to be an important regulator of renal histoarchitecture.
The molecular regulation of kidney development and ureteric branching morphogenesis has been extensively studied in vitro [1–8]. Whole metanephric organ culture, in which the whole mouse or rat metanephros is explanted just after the ureteric bud has invaded the metanephric mesenchyme and is cultured for up to 7–8 days, is a very powerful and the most widely used in vitro model of metanephric development. Both branching of the ureteric epithelium, and mesenchyme to epithelium conversion forming nephrons, occur in metanephric organ culture. Soluble factors or their inhibitors can be added to the culture medium in order to examine their roles in renal development.
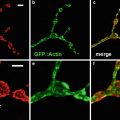
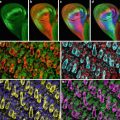
Despite the power of metanephric organ culture as a model for studying the roles of specific molecules in kidney development, there have been relatively few quantitative studies of ureteric branching morphogenesis in vitro. To date, quantitative analysis of ureteric duct growth and branching has been mostly limited to measurements in two dimensions. Although providing some indication of changes in ureteric growth, these measurements are inaccurate in that they do not take into account the third dimension of the growing ureteric tree. We have developed image processing and analysis algorithms which allow us to quantitatively, objectively, and accurately measure ureteric branching morphogenesis in the cultured mouse kidney in 3D. This method has allowed us to detect previously unreported asymmetric growth of the mouse ureteric tree [9]. The method also allows us to measure individual branch lengths as well as the length of specific branch “generations.” In addition, this technique allows the development of growth curves, identification of the relationship (if any) between the lengths of adjacent branches, and the development of mathematical models of ureteric growth. This method has been used to examine the normal growth of the mouse ureteric tree in vitro [10]. Our data suggest there is tight regulatory control of ureteric branching which ultimately results in the defined pattern of branching morphogenesis that is unique to the kidney. This technique now also allows the detection of subtle differences in the growth and pattern of branching in the presence of various soluble factors as well as the branching phenotypes in knockout and heterozygous mice [9, 11]. Furthermore, this method is now being used to study the growth of living ureteric trees in vitro, utilizing the fluorescent ureteric trees (Fig. 1) of Hoxb7/GFP transgenic mice [12]. Quantitative analysis of a tree on multiple occasions provides a powerful approach to studies of ureteric tree growth rates, generation data, and remodeling under experimental conditions. This chapter describes techniques for quantitative analysis of the ureteric tree in developing mouse kidneys in vitro. Separate protocols are provided for 3D and 4D imaging and analysis.


Fig. 1
Photomicrographs of in vivo Hoxb7/GFP kidneys at several developmental time points. (a) E11.5, (b) E13.5, (c) E15.5, (d) E17.5 (not to scale)
2 Materials
2.1 Metanephric Dissection
1.
Fine forceps (Dumostar Biology, straight, superfine point forceps, 110 mm, ProSciTech, Thuringowa Qld Australia).
2.
Entomological pins, E179, B3; 0.0076 × 15 mm (Australian Entomological Supplies, Bangalow, NSW, Australia).
3.
Sterile phosphate buffered saline (PBS): dissolve 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2PO4, and 0.24 g of KH2PO4 in 800 ml distilled H2O. Adjust pH to 7.4 with HCl. Adjust volume to 1 L with additional distilled H2O. Sterilize by autoclaving.
4.
60 mm × 15 mm tissue culture dish (Becton Dickinson Labware, NJ, USA) with a layer of Sylgard-silicone elastomer (Dow Corning Corp, MI, USA).
2.2 Metanephric Culture and Fixation
1.
Serum-free culture medium (see Note 1): Dulbecco’s Modified Eagle’s Medium (DMEM): Ham’s F12 liquid medium (Trace Biosciences, Castle Hill, NSW, Australia) supplemented with 5 mg/ml transferrin (Sigma-Aldrich Pty. Ltd., Castle Hill, Australia), 12.9 μl/ml l-glutamine (Trace Biosciences), and 100 μg/ml penicillin/100 U/ml streptomycin (Trace Biosciences).
2.
3.0 μm polycarbonate transfilter membrane insert (Transwell, Corning Costar Corporation, Cambridge, Massachusetts, USA).
OR
3.0 μm × 13 mm Poretics polycarbonate membrane filters (GE Osmonics Inc, Minnetonka, MN, USA).
3.
24-well culture plate (Becton Dickinson, Franklin Lakes, New Jersey, USA).
4.
35 mm × 10 mm tissue culture dish (Becton Dickinson, Franklin Lakes, New Jersey, USA).
5.
Fixative: ice cold 100 % methanol.
OR
4 % paraformaldehyde (PFA) in PBS at 4 °C: dissolve 2 g PFA and 2 NaOH pellets in 50 ml PBS, pH to 7.4 (make up fresh each time).
2.3 Immunolabeling
1.
24-well culture plate.
2.
PBS.
3.
Blocking solution: 5 % fetal calf serum (FCS) in PBS, make fresh for each use.
4.
Antibody diluent: 4.25 g NaCl, 0.2675 g Na2HPO4, 0.0975 g NaH2PO4∙2H2O, and 2.5 ml 10 % sodium azide in 250 ml dH2O, pH to 7.1.
5.
Primary antisera: monoclonal mouse anti-Calbindin-D28K (Sigma Chemical Co., St. Louis, Missouri, MO, USA) at 1:200 in antibody diluent. Secondary antisera: Alexa 488 goat anti-mouse IgG (Molecular Probes, Eugene, Oregon, USA) at a dilution of 1:100 in antibody diluent. Both these antibodies can be aliquoted prior to dilution and stored at −20 °C. Once thawed, antibody can be stored at 4 °C for approximately 1 month.
7.
Mounting medium: Glycerol in PBS with sodium azide (Sigma Diagnostics, St. Louis, Missouri, MO, USA).
8.
Cavity slide sealer: clear nail polish.
3 Methods
3.1 3D Analysis of Ureteric Tree Growth
Metanephric Dissection
1.
Mice or rats need to be time-mated. The presence of a vaginal plug designates that mating has occurred. Embryonic day 0 (E0) is defined as midnight during the night of mating. Pregnant females are sacrificed by cervical dislocation and embryos removed on E12 (mouse) or E14 (rat).
3.
Decapitate and pin embryo through first the neck region and then the base of the tail to the layer of Sylgard-silicone elastomer within the Petri dish (see Note 4).
4.
Using fine forceps, remove abdominal contents and discard.
5.
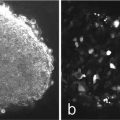
Using fine forceps, remove complete urogenital ridge including metanephric kidneys (Fig. 2).


Fig. 2
Urogenital ridge of E12 mouse embryo. Arrows metanephroi; Asterisk Wolffian ducts
6.
Using forceps, pry away metanephric kidneys from urogenital ridge and pipette into sterile serum-free culture medium warmed to 37 °C.
Metanephric Culture and Fixation
1.
Wash metanephroi twice in serum-free culture medium warmed to 37 °C and pipette onto 3.0 μm polycarbonate transfilter membrane insert sitting on 350 μl of culture medium/well in a 24-well plate.
OR
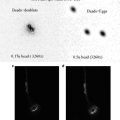
Pipette onto a 3.0 μm polycarbonate filter membrane floating in a well in a 24 well plate containing ≥350 μl culture media (Fig. 3a).


Fig. 3
Phase contrast photomicrographs of an E12 mouse metanephros in culture. (a) day 0, (b) Day 1, (c) Day 2. Bar = 250 μm
3.
At the end of the culture period fix metanephroi in ice cold methanol for a minimum of 15 min at −20 °C (see Notes 5 and 6).
Immunolabeling (Not Required for HOXB7/GFP-Labeled Metanephroi)
1.




If using polycarbonate inserts use a scalpel blade to remove the membrane from the bottom of the insert.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree