and William Sullivan1
(1)
Molecular and Cellular Biology, University of California, Santa Cruz, CA, USA
Abstract
The model organism Drosophila melanogaster is particularly well suited for live image analysis. The availability of GFP transgenic flies and a wide array of fluorescent probes, in conjunction with laser scanning confocal microscopy, allow us to image multiple aspects of the cell cycle simultaneously. Confocal microscopy provides the sensitivity and resolution to observe the dynamics of specific cellular events in real time. For example, GFP-histone and rhodamine-labeled tubulin enable one to follow specific nuclear and cytoskeletal events including nuclear envelope formation, nuclear envelope breakdown, spindle formation, centrosome duplication, separation and migration, chromosomes condensation, and segregation. This analysis permits a detailed morphological and temporal description of nuclear and cytoskeletal events in normal or drug-injected embryos.
Key words
Drosophila MicroinjectionDrugs1 Introduction
Advances in fluorescent microscopy and the development of sensitive highly specific fluorescent probes have considerably advanced our ability to observe subcellular events in living organisms. Laser scanning confocal microscopy provides the sensitivity and resolution to observe the dynamics of specific cellular events in real time. GFP technology and other technologies have provided the community of cell biologists with a wealth of fluorescent probes to follow countless cellular events. These techniques have proven especially powerful in producing detailed analysis of mutant phenotypes.
The model organism Drosophila melanogaster is particularly well suited for live image analysis. In Drosophila, the events of early embryogenesis have been characterized in detail using both fixed and live analysis [1–4]. The initial nuclear divisions are rapid (10–20 min long), synchronous, and syncytial. The first seven rounds of nuclear division occur in the interior of the embryo. During nuclear cycles 8 through 10, the majority of the nuclei migrate to the cortex where they undergo four more rounds of division before cellularizing during a prolonged interphase of nuclear cycle 14. These divisions alternate between M and S with highly abbreviated G1 and G2 phases. Importantly, these cell cycles are regulated in the same fashion as more conventional cell cycles. In spite of many statements to the contrary, these cycles possess robust S-phase, DNA damage, and mitotic cell cycle checkpoints [5–8]. A unique feature of these cycles is that they do not require zygotic transcription and do not undergo apoptosis [9]. As will be described below, this is advantageous because the posttranslational effects of drugs and inhibitors on the cell cycle can be readily determined. Finally, multiple rounds of nuclear divisions can be imaged on a single focal plane, as the cortical divisions occur in a monolayer [7, 10].
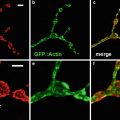
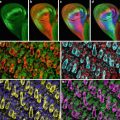
The availability of GFP transgenic flies and a wide array of fluorescent probes, in conjunction with confocal microscopy, allow us to image multiple aspects of the cell cycle simultaneously. For example, GFP-histone and rhodamine-labeled tubulin enable one to follow specific nuclear and cytoskeletal events including nuclear envelope formation, nuclear envelope breakdown, spindle formation, centrosome duplication, separation and migration, chromosomes condensation, and segregation. Figure 1 illustrates the power of this approach. Panel A shows nuclear envelope formation (NEF), which occurs at the end of telophase and is characterized by the exclusion of tubulin (in red) from the nuclei (in green). As shown in panel B, the appearance of bright histone-GFP dots in the nuclei marks the initiation of chromosome condensation (ICC). Following ICC, the chromosomes become more condensed and pull away from the nuclear envelope, thus defining a second distinct phase of chromosomes condensation (CC2). Nuclear envelope breakdown (NEB) marks the beginning of prophase. The entrance of monomeric tubulin in the nuclear space characterizes this stage (D). Entrance into metaphase is marked by the formation of a bipolar spindle, decrease in asters, and alignment of condensed chromosomes on the metaphase plate (Panel E). In panel F, elongation of the metaphase spindle, in addition to segregation of chromosomes, indicates the initiation of anaphase (IA). Equally important to the analysis of the morphological events is that the abbreviated cycles enable one to precisely time the interval between each event. Another important attribute of analyzing the cortical syncytial cycles is that it is possible to inject inhibitors at precisely timed stages of the cell cycle. For example, if we want to time the S-phase in a colchicine-injected embryo, it is necessary to inject the drug after anaphase when chromosomes have already separated, and there is no risk of activation of the spindle checkpoint. This analysis permits a detailed morphological and temporal description of nuclear and cytoskeletal events in normal or in drug-treated embryos. It is even possible to identify drugs that, when injected into the embryo, do not show any visible phenotype but do alter the relative timing of cell cycle events.


Fig. 1
Images of nuclear and cytoskeletal events of a syncytial Drosophila embryo bearing the histone-GFP construct and injected with fluorescently labeled tubulin
2 Materials
2.1 Embryo Preparation
1.
Mechanical needle puller (Model P-30, Sutter Instrument Co.).
2.
Injection apparatus.
3.
75 mm capillary tubing with an outer and inner diameter of 1.21 and 0.90 mm, respectively (Drummond Scientific Broomall, PA cat. no. N-51 A).
4.
Atoxic clay mount in a Petri dish.
5.
10 μl syringe (Hamilton).
6.
Microscope slides (Fisher).
7.
22 × 50 mm2 coverslips (Fisher).
8.
22 × 22 mm2 coverslips (Fisher).
9.
Tape (Fisher).
10.
Dissecting forceps (Dumont # 5).
11.
Halocarbon oil (Series 77, CAS no. 9002-83-9, Halocarbon Products Corp.).
12.
Heptane (EM Science).
13.
Double-stick tape (3 M).
14.
Refrigerated microcentrifuge.
15.
Drierite granular desiccant.
16.
Lids from 35 × 10-mm disposable plastic tissue culture dishes (Falcon).
17.
Plastic “flies collection” bottles (Applied Scientific).
2.2 Visualization and Drug Treatment
1.
Inverted microscope with confocal imaging system.
2.
Fluorescently labeled tubulin: Molecular Probes (Eugene, OR), or Cytoskeleton, stored at −80 °C in 2 μl aliquots of 20 μg each.
3.
GFP-Histone fly stock kindly provided by Robert Saint.
3 Methods
3.1 Preparation of Glue
To allow the embryos to remain stably attached to the cover slip during the period of observation, prepare a concentrated embryo adhesive: 10 ml of heptane are added to a 50 ml conical tube containing 12 in. of double-stick tape, and the tube is allowed to rotate overnight. The resulting solution is aliquoted in 1.5 ml microcentrifuge tubes and then centrifuged at 14,000 rpm (15,800 × g) for 10–15 min to remove the particulate matter. The clear solution is then transferred to a tightly closed container to avoid evaporation. To make a working solution, the concentrated glue should be diluted roughly five times with heptane.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree