(1)
Department of Zoology, University of Coimbra, Coimbra, Portugal
(2)
Department of Biomedical Sciences, Mercer University School of Medicine, Savannah, GA, USA
(3)
Department of Anatomy, Microbiology and Pathology, University of Minnesota School of Medicine, Duluth, MN, USA
Abstract
Cell behavior is significantly different in two-dimensional and three-dimensional culture conditions, and a number of methods have been developed to establish and study three-dimensional cellular arrays in vitro. When grown under nonadherent conditions, many types of cells form structures called multicellular spheroids (MCSs), which have been popular models to study cell behavior in a three-dimensional environment. The histoarchitecture of MCSs derived from malignant cells resembles that of tumors, and there is rapidly increasing interest in using these structures to more accurately understand the dynamics of cancer cells in situ, including their responses to chemotherapeutics. Confocal microscopy is an extremely useful method to investigate cell behavior in MCSs due to its ability to more clearly image fluorescent probes at some depth in three-dimensional structures. This chapter describes some basic approaches toward visualizing a variety of fluorescent probes in MCSs.
Key words
Multicellular spheroidVital imagingFluorescent labelingMitochondriaNucleiAn erratum to this chapter can be found at http://dx.doi.org/10.1007/978-1-60761-847-8_18
1 Introduction
An intriguing approach toward assessing cell function by microscopical methods is the use of multicellular spheroids (MCSs) as experimental material. Although individual cells in monolayer culture generally yield the best resolution and clearest imaging, their behavior can be quite different from that of cells existing in their normal milieu, namely, surrounded by other cells and/or extracellular matrix. MCSs can be used to strike a balance between studying cell behavior under more natural conditions while allowing for a significantly better level of imaging that is achievable with an actual tissue or organ. MCSs have been constructed from untransformed cells in order to study tissue organization and function in vitro as well as from transformed cell lines in order to study malignant cell behavior in a more tumor-like setting [1–3]. This chapter deals solely with the use of transformed cell lines to study cell behavior in living MCSs treated with various vital imaging probes.
Similar to in situ solid tumors, MCSs derived from transformed cell lines display structural heterogeneity and exhibit a range of microenvironments with respect to oxygen, glucose, pH, and other gradients. A primary difference between the two reflects the vascularization of in situ tumors, but in both cases, gradients of diffusible material are dominant factors in the establishment of functionally distinct regions. In general, all of the cells in smaller MCSs may remain viable, but larger MCSs characteristically consist of an outer shell of proliferating cells, an intermediate layer of quiescent cells, and a core of moribund or dead cells [4, 5]. This architecture is usually presumed to reflect a low internal oxygen pressure, but the actual mechanisms influencing survival, growth, and death in an MCS appear to be more complex [3].
The behavior of cells in MCSs is usually quite different than that of the same cell line grown as an adherent monolayer. Notably, MCSs commonly exhibit increased resistance to anticancer agents compared to monolayer cultures [6–9]. This effect has been termed multicellular-mediated resistance [10] and arises from multiple mechanisms. For example, the expression of integrins, cadherins, cyclin-dependent kinase inhibitors, and other proteins can all be altered in three-dimensional cultures of MCSs compared to their monolayer counterparts [9, 11–14]. Furthermore, mathematical modeling studies indicate that drug penetration is a critical feature of the increased resistance of MCSs to chemotherapeutic treatment [15] and the expression of P-glycoprotein can influence the distribution of cell-permeant xenobiotic compounds within MCSs [16–18]. Together, these observations have led to compelling arguments for the superiority of MCSs over monolayer cultures for in vitro drug testing [3, 19, 20]. Germain to this chapter, MCSs ranging from a few dozen to many hundreds of cells in size are also ideal platforms to showcase the advantages of confocal microscopy over routine epifluorescence microscopy in imaging thicker specimens and multicellular aggregates.
2 Materials
2.1 Cell Lines and Culture Conditions
Most of our studies have so far been conducted using various human and mouse cancer cell lines because of the ease in maintaining continuous cultures for experimental material. Some cell lines readily form well-organized MCSs when maintained in suspension, whereas others are not suitable in that lack of adhesion to a surface triggers a type of cell death termed anoikis. In addition, some cell lines simply do not adhere well enough to each other in suspension to form a well-organized MCS (e.g., PC-3 prostate cancer cells and H9c2 myoblasts). In some cell lines such as MCF-7 breast cancer cells, MCS formation is sensitive to specific culture conditions [3], and the best approach for a particular cell line can either be suggested from the literature or derived empirically. Overall, the form, function, ontogeny, senescence, and death of MCSs are composite reflections of both the cell line and the culture conditions.
One of the most straightforward approaches to producing MCSs is to simply culture dispersed cells in suspension; MCSs can subsequently form either from an aggregation of suspended cells, by the cohesion of daughter cells from a mitotically active founder cell(s) or a combination of both processes. A number of different methods to keep cells in suspension during MCS formation have been described and include the use of spinner flasks, roller tubes, plating cells on bacteriological-grade culture dishes, or adding a cell suspension to a dish that has been coated with agarose to prevent attachment. We normally use bacteriological-grade dishes to establish MCSs in static cultures, which give acceptable results for most of the cell lines we commonly use (see Note 1). We have adapted most of the cell lines we use in our laboratory to a standard cell culture medium consisting of Dulbecco’s modification of Eagle’s medium (DMEM; GIBCO Biologicals, Grand Island, N.Y.), 1 mM sodium pyruvate, and 7.5 % Fetal Clone III (FCIII; HyClone, Logan, UT).
2.2 Fluorescent Probes
Three different examples of vital imaging approaches using MCSs are discussed here, namely, (a) imaging cell viability and nuclear and mitochondrial dynamics using calcein-AM, Hoechst dyes, and tetramethylrhodamine methyl ester (TMRM); (b) imaging the uptake and distribution of certain drugs that are intrinsically fluorescent; and (c) using cells transfected with fluorescent reporter constructs to seed MCSs. If the experiment only requires a relatively short imaging period (e.g., 10 min or less), fluorescent probes can be added directly to the medium used to culture MCSs. However, most media intended for use in a CO2 incubator soon become excessively basic when kept outside of the incubator, as occurs, for example, during time-lapse studies on the stage of a confocal microscope. For these experiments, the media can be modified to maintain a neutral pH outside of an incubator (see Note 2).
1.
Tetramethylrhodamine methyl ester (TMRM; catalog no. T668, Molecular Probes, Eugene, OR): A mitochondrial membrane potential-sensitive probe. TMRM predominantly labels polarized mitochondria (i.e., mitochondria with transmembrane electric potentials generated by respiration) a bright red color. A primary stock solution of 10 mg/ml is prepared by diluting the contents of a 25 mg vial with 2.5 ml dimethyl sulfoxide (DMSO). The resulting solution will have a deep red color and should be divided into small aliquots and kept frozen and protected from light (see Note 3). When needed for experiments, a working stock solution is prepared by diluting the primary stock 1:100 in culture medium lacking serum (e.g., add 1 μl of 10 mg/ml TMRM to 99 μl DMEM). Add 1.5 μl of working stock/ml of cell culture medium (containing MCSs) and incubate for about 1 h in a cell culture incubator prior to imaging. The working solution can be stored in a freezer and thawed when needed; it can withstand a few freeze-thaw cycles over about 7–10 days. TMRM can be used to assess differences in the morphology of functional mitochondrial as well as the magnitude of the mitochondrial transmembrane electric potential (see Note 4). Depending on the experimental conditions and probe concentration, TMRM can be used in a “self-quenching mode,” where a decrease in membrane potential will initially result in increased TMRM fluorescence or in the “non-quenching” mode, where a decreased mitochondrial membrane potential will result in decreased probe fluorescence [21, 22]. The ideal concentration for visualizing mitochondria in MCSs made from different cell lines can be empirically determined; a final concentration of between 100 and 200 nM has worked well for all of the cell lines we have examined to date.
2.
Calcein-AM (catalog no. C1430, Molecular Probes): An esterase substrate that living cells hydrolyze to yield calcein, a membrane impermeant, polyanionic fluorescein derivative. A stock solution of 10 mM is prepared by diluting the contents of a 1 mg vial with 100 μl DMSO. The resulting solution is colorless and should be divided into small aliquots and stored as described for TMRM. When needed for experiments, a working solution should be prepared by performing a 1:10 dilution in culture medium lacking serum (e.g., add 10 μl of 10 mM calcein-AM to 90 μl DMEM). 2.5 μl/ml of the working stock is added to culture media containing MCSs for about 1 h prior to imaging. Calcein-AM begins to be hydrolyzed once diluted in aqueous solution, so it is best to make up just the amount of working stock required for each experiment (see Note 3). This is a useful probe to assess cell morphology and viability, as calcein is retained in living cells upon cleavage of the acetoxy-methylester moiety by cellular esterases [23].
3.
Hoechst (bisBenzamide): A DNA intercalator that inserts into A-T rich regions of chromatin and labels nuclei blue. A stock solution of Hoechst 33342 (Catalog no. B 2261, Sigma Chemical Co., St. Louis, MO) of 1 mg/ml is made in water and stored in the dark at 4 °C. Other varieties of Hoechst are available, but 33342 is reported to penetrate living cells somewhat more readily than, for example, 33258. Hoechst is a suspected mutagen, and the use of gloves when handling is advised.
4.
Sanguinarine: A benzophenanthridine alkaloid that binds to C-G rich regions of DNA. A number of studies have focused on the ability of sanguinarine to induce malignant cell death by triggering apoptosis [24–26]. Sanguinarine has been reported to induce a bimodal pattern of cell death, with lower concentrations triggering caspase activation and apoptosis, and higher concentrations causing oncosis, or “blistering cell death” in the absence of caspase activation [26, 27]. Sanguinarine can be visualized with a blue Krypton-Argon laser (488 nm excitation line) in conjunction with either a green 515/30 nm band pass filter or a red 605/75 band pass filter (see Note 5), and previous studies showed that sanguinarine rapidly accumulates within the nucleus [28]. A 50 mM stock solution of sanguinarine (sanguinarine chloride, catalog no. S 5890, Sigma) is made in DMSO, aliquoted into small volumes, and stored in the freezer. A working stock solution of 500 μM is made by diluting the 50 mM stock 1:100 in serum-free culture medium just before use and 2–40 μl/ml added to make final concentrations of between 1 and 20 μM, depending on the experiment. The 50 mM stock solution in DMSO can be freeze-thawed a number of times and is stable for a few weeks.
5.
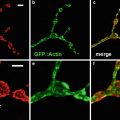
Spectral variants of green fluorescent protein and DsRed: Since the development of green fluorescent protein (GFP) as a reporter molecule, a number of improvements have been made in the use of GFP and related molecules for cell and molecular biological studies. Newer versions of these probes have been engineered to be more photostable, less self-aggregating, and more tolerant of N-terminal fusions [29]. For the work shown in this chapter, we used the plasmid phrGFP-1 expressing the humanized Vitality hrGFP Renilla reniforms green fluorescent protein from the CMV promoter (Stratagene Inc., La Jolla, CA) and the plasmid pcDNAtdTomato (tdTomato open reading frame was a generous gift from Dr. Roger Tsien, University of California at San Diego and is commercially available from Clontech Laboratories, Inc., Mountain View, CA). This vector expresses the tdTomato fluorescent protein, a modified DsRed Discosoma sp. tandem-dimer fluorescent protein.
3 Methods
3.1 Construction of MCSs
We routinely make MCSs from P19 mouse embryonal carcinoma cells, K1735-M2 mouse melanoma cells, and LNCaP human prostate cancer cells. The easiest approach for these cell lines is to maintain them as monolayers in tissue culture-grade dishes and to use cells to seed MCSs during passaging using routine phosphate-buffered saline (PBS)/EDTA (ethylenediaminetetraacetic acid)-trypsin protocols. For example, we use the following steps to construct MCSs from these cell lines:
1.
Trypsinize cultures when they are between 50 and 75 % confluency; count cells using a trypan blue solution and hemocytometer by standard methods.
2.
Adjust cell density to about 5 × 104/ml in fresh media (e.g., DMEM/7.5 % FCIII) and add to a bacteriological-grade (“nonsticky”) Petri dish. A single 60 mm diameter dish will yield enough MCSs for a small number of vital imaging experiments; cultures can easily be scaled up (e.g., multiple 100 mm diameter dishes can be seeded).
3.
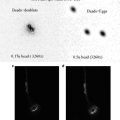
Incubate cells for a few days in a regular CO2 incubator at 37 °C. During the first few days, MCSs are small and composed of healthy, viable cells. By 5 or 6 days, they are much larger and begin to contain a core of dead and dying cells, surrounded by a layer of living cells. For vital labeling and imaging, a number of the MCSs are removed from the bacteriological plates and transferred to a suitable labeling/imaging chamber (see below), and the remainder returned to the incubator. At this time, MCSs can be size-selected if desired, using a low-power inverted or dissecting microscope.
4.
MCSs can be maintained in the bacteriological plates for quite a few days with periodic aspiration and replacement of the culture media. We simply remove old media with a sterile Pasteur pipette connected to a vacuum source; a few MCSs are lost during this procedure, but most can be left in the dish if one is reasonably careful during aspiration (they are visible and can be avoided). MCSs continually grow and change while cultured, so MCSs of the same age should be used for comparison purposes across experiments.
3.2 Fluorescent Labeling of Living MCSs
The first step is to remove the desired number of spheroids from the bacteriological-grade dishes and transfer them to an appropriate imaging chamber. Because our confocal unit is mounted on an inverted microscope, we routinely use glass-bottom 30 mm diameter culture dishes from Mat-Tek (Ashland, MA) for this purpose. For example, add 2.4 ml media (e.g., DMEM/7.5 % FCIII) to a 30 mm diameter dish and then transfer 100 μl media containing MCSs from the bacteriological dish. MCSs are usually easily visualized by the naked eye, and we simply use a micropipette and a sterile tip to remove them from the bacteriological plate.
Get Clinical Tree app for offline access

1.
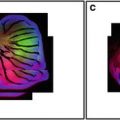
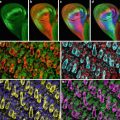
For studies of cell viability and mitochondrial morphology, we use a cocktail of Hoechst, TMRM, and calcein-AM. While swirling the medium in a glass-bottom dish containing MCSs, add 5 μl/ml Hoechst 33342 (1 mg/ml stock), 1.5 μl/ml diluted TMRM stock (100 μg/ml in DMEM), and 2.5 μl/ml diluted calcein-AM stock (1 mM in DMEM). Replace the dish in a CO2 incubator for about 1 h prior to imaging. Under these conditions, the fluorescent probes penetrate and can be visualized to a depth of about 20 μM (Figs. 1, 2, and 3).