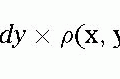Congenital Heart Disease
Robert W. W. Biederman
The use of cardiovascular magnetic resonance (CMR) to evaluate congenital heart disease (CHD) has revolutionized the study and practice of CHD. It is almost as if CMR was developed primarily to assess CHD, with advantages for simple and complex, embryologically disparate disease processes in, typically younger, sick patients. Aspects that are ideal for this population include noninvasive, nonionizing radiation; no obligate need for contrast; ability to quantitate in highly accurate and reproducible manner both flow and velocity; visualization of ventriculoarterial conduits; assessing relationships between vascular structures that are typically not seen well on x-ray angiography; lack of requirement of acoustic window; and ability to visualize in any plane (see Fig. 14-1).
Hemodynamic assessment in a noninvasive manner is a key advantage that has been well recognized for detection and quantification of stenotic jets. Jets are characterized in terms of peak and mean gradient, and the temporal relationship of the jet to cardiac events can be assessed in a manner analogous to echocardiography, except that it can be done in an inherently three-dimensional manner. True quantitation of regurgitant jets, however, is not something that any other technique offers. CMR, through phase velocity mapping (PVM) allows calculation of the amount of blood flow crossing a selected plane, independent of relative orientation (see Fig. 14-2). For example, the same quantitation procedure can be performed in the setting of one valve possessing both a systolic stenotic jet and a diastolic regurgitant jet.
The ability to quantitate intracardiac shunts is well performed by CMR. Conventionally, echocardiographic measures cannot quantitate shunts well, and when attempted, these approaches are reliant on estimating velocity time integrals, which inherently assume that a parabolic flow is present. Similar arguments apply to the assessment of shunts in the catheterization suite where thermistor methods suffer in the presence of regurgitant lesions, whereas oximetry measurements suffer from a lack of specificity when there is >6% change in oxygen saturation levels. Fundamentally, this limits the ability to detect small shunts, which, although they may not be clinically significant, can become significant over time.
Typically, the CMR patient has been referred from a prior imaging modality in which the diagnosis was unclear. At our institution, echocardiography is the most often used test, followed by x-ray imaging and lastly, computed tomography (CT). Clarification of the disease process, relating an observation to the great vessels, evaluation of situs, evaluations of potential ventriculoarterial connection or intracardiac shunts are usually the indications to consider CMR (see Fig. 14-3).
A wide range of CMR imaging sequences are useful for patients with CHD. For a strategy that permits large amount information to be gleaned in a short time, to allow interpretation and permitting flexible planning, the double inversion recovery (DIR) sequence has proved to be very robust. When images are acquired in an axial view, anatomic relationships can be determined in a rapid manner, because the body is most commonly considered in this orientation. Relating the great vessels to the ventricles is easily performed in this plane, as is establishing the general relationships that exist between the atria and the ventricles, and additionally, some intracardiac communications can be assessed. The coronal view albeit less traditional, because it is not obtainable by conventional image modalities, reveals anatomic relationships in a superb manner, serving to delineate vascular and myocardial structures effectively in three dimensions (3D), when relating the series of 2D planes.
Defining situs is relatively easy when image quality is high. Situs solitus is the normal position, with the morphologic thin, smooth walled right atrium (RA) with its broad-based appendage and crista terminalis positioned to the right of the left atrium (LA), with its thin, miterlike, LA appendage. When reversed, the condition is referred
to as situs inversus, and when ambiguous, it is referred to as atrial isomerism. This latter condition is associated with the visceral heterotaxy syndromes (see Fig. 14-4). Describing bronchial anatomy to identify the right and left bronchi is also quite helpful, especially when the lung isomerism is present. Right isomerism is associated with asplenia, whereas left isomerism is associated with polysplenia. Each condition has many associated atrioventricular (AV) connections. See Chapter 7 for more details.
to as situs inversus, and when ambiguous, it is referred to as atrial isomerism. This latter condition is associated with the visceral heterotaxy syndromes (see Fig. 14-4). Describing bronchial anatomy to identify the right and left bronchi is also quite helpful, especially when the lung isomerism is present. Right isomerism is associated with asplenia, whereas left isomerism is associated with polysplenia. Each condition has many associated atrioventricular (AV) connections. See Chapter 7 for more details.
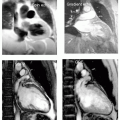
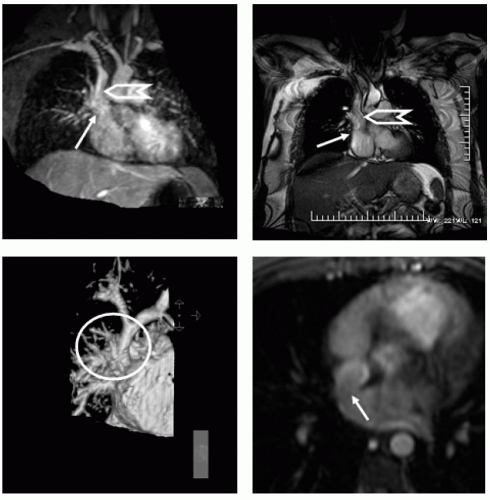 FIGURE 14-1 Top panels show a limited region maximum intensity projection (MIP) (left). A steady state free precession (SSFP) (right) image acquired in the coronal projection at the level of the superior vena cava (SVC) demonstrates a confluence of the right upper and middle right pulmonary veins entering into the SVC/high right atrial (RA) junction. The lower panel images show a 3D surface rendition (left panel) of the magnetic resonance angiography (MRA) for the defect, demonstrating in the surface reconstructed view manually timed for the main pulmonary artery. The right panel shows the “broken ring” sign depicted by the arrow as the classic axial image that is diagnostic of a communication between the SVC and RA with a defect in the posterior inner atrial system. Images relate to case study 1. |
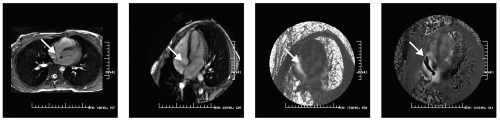 FIGURE 14-2 A selected multislice steady state free precession (SSFP) sequence and a four-chamber view of an ostium secundum defect (far left and left) with phase velocity mapping (PVM) set low to a Venc of 50 cm/second shown in the left-to-right and anterior-to-posterior direction (right and far right) demonstrating the “Doppler-like” capability of cardiovascular magnetic resonance (CMR) with PVM. Quantitation was also performed to noninvasively determine the intracardiac shunt (Qp:Qs). Images relate to case study 2. |
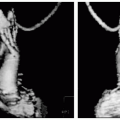
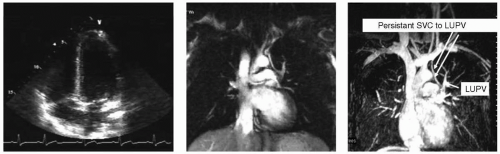 FIGURE 14-3 Transthoracic imaging just before near simultaneous opacification of the right ventricular (RV) and left ventricular (LV) chambers by saline contrast (left). Middle image shows a single steady state free precession (SSFP) slice hinting at anomalous connection to the left upper pulmonary vein (LUPV)and confirmed by a temporal acquired maximum intensity projection (MIP) (arrows) (right). SVC, superior vena cava. Images relate to case study 3. (Best seen on DVD.) |
Evaluation of pulmonary vein drainage is exceptionally well defined by CMR, as many combinations and anatomic normal variants are increasingly being recognized as clinically important (see Fig. 14-5). The axial plane generally allows recognition of the insertion point of the veins into the posterior LA. Usually, acquisition of a coronal plane permits unequivocal detection of the veins, and more importantly, when aberrant drainage is suspected, this plane permits delineation of a number of insertions into other vascular territories, including infradiaphragmatic drainage, which is generally not detectable by other imaging modalities. In many cases in which right ventricular (RV) dilation is present, as seen on transthoracic echocardiography (TTE), and despite referral for transesophageal echocardiography (TEE) in which, “all four pulmonary veins are identified and enter into LA” CMR often detects a third right pulmonary vein (expected in many cases due to the smaller right middle pulmonary vein position that can be mistaken for the right lower pulmonary vein or right upper pulmonary vein). In these cases, often a sinus venosum defect is seen on axial DIR images, which easily detects a “broken ring sign” in which the posterior superior vena cava (SVC) is not completely closed and a defect is seen where the right upper pulmonary vein drains into the junction between the SVC and RA (see Fig. 14-1). Anomalous return, either partial in adults, or total in young children has been quite well described by CMR.
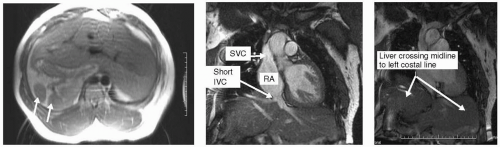 FIGURE 14-4 Double inversion recovery (DIR) on left with steady state free precession (SSFP) in coronal projections demonstrating the heterotaxy syndrome. IVC, inferior vena cava; SVC, superior vena cava; RA, right atrium. Images relate to case study 4. |
CASE STUDIES
Case Study 1.
A persistent left superior vena cava (SVC) is visualized in a 46-year-old woman with shortness of breath (SOB), suggesting the possibility of a previously unrecognized unroofed coronary sinus atrial septal defects (ASD). However, a confluence of right upper pulmonary veins entering the high right atrium/proximal right SVC is seen (see Fig. 14-1), consistent with a sinus venosum defect. The sinus venosum defect is seen in a coronal image depicting the entry site into the RA (arrow) and right SVC (chevron). The bottom left image is a 3D surface from the magnetic resonance angiography (MRA) of a 29-year-old woman in whom a murmur was heard and an echocardiography was performed demonstrating right ventricular (RV) enlargement, which prompted the CMR. A sinus venosus defect was seen for which she underwent surgical baffle from the SVC/RA to the LA redirecting pulmonary flow to the LA and venous return to the RA.
Case Study 2.
A 35-year-old woman presents with SOB. A CMR demonstrates a moderate-sized ostium secundum ASD (see Fig. 14-2). Various PVMs convincingly demonstrate the magnitude of the shunt which was measured as a Qp:Qs = 2.1 : 1 for which she underwent percutaneous placement of a closure device. SSFP and PVM were all that was necessary to accurately define the physiology. If an intracardiac shunt is suspected by SSFP imaging, confirmation can be obtained by switching to a gradient-recalled echo (GRE) sequence and increasing the echo time (TE) to 8.5 to 10 milliseconds. Further confirmation and quantification can be obtained using PVM as shown in the right panels. Often, a more dramatic visualization can be demonstrated through PVM than can be achieved by a dephasing artifact.
Case Study 3.
An 18-year-old black male football player presents with SOB. Routine echocardiography reveals markedly positive bubble study (left arm IV). TEE performed demonstrates no bubbles crossing. Repeat TTE performed again show copious bubble immediately crossing from the RA to the LA. Unknown diagnosis prompts CMR (see Fig. 14-3). On the basis of presentation, a coronal SSFP was performed (mid panel) and suggested the diagnosis. Temporal MRAfrom the left arm shows two mechanisms of venous return into the heart. Note the exquisite anatomic detail depicted, demonstrating both anatomy and physiology with the transit of contrast over time and space. This is diagnostic of a very rare anomalous left upper pulmonary vein draining into the cardinal vein (“Partially Partial Anomalous”).
Case Study 4.
This 32-year-old woman presents for evaluation of dyspnea. Routine CMR reveals an incidental finding of an interrupted inferior vena cava (IVC) (see Fig. 14-4). Note the multiple circular densities (see Fig. 14-4, left panel, arrows) indicative polysplenia, an often associated finding. This can be part of the Heterotaxy syndrome. Note the large SVC (middle panel) from the rerouted azygous return through which the mesenteric venous flow destined for the RA is rerouted. This is a benign variant for which, in the absence of imaging, she would be oblivious to her anomaly.
Case Study 5.
Axial-SSFP images revealing anomalous right upper pulmonary venous (RUPV) drainage into the mid SVC in a 41-year-old woman with palpitations and mild dyspnea on exertion (DOE) (see Fig. 14-5). A sinus venosus anomaly is differentiated, as the RUPV drains into the junction of the high RA and low SVC. Note the confluence of two pulmonary veins into SVC; which are very important for complete surgical repair, in addition to identification of any and all anomalous drainage into the SVC because the surgeon may only be expecting to correct one vein.
Case Study 6.
A 41-year-old white man with diagnosis of I-transposition since a child, and told he would never be normal. Multiple subsequent angiograms and TTEs and TEEs had been performed over the ensuing years, each reinforcing the last, saying that he had congenitally corrected transposition and eventually his heart would fail. Referred to us almost by accident, he underwent CMR (see Fig. 14-6). The RV moderator band is on the rightward and anterior trabeculated ventricle, whereas the leftward nontrabeculated ventricle AV valve plane is displaced atrially relative to the right AV valve plane (upper panels). There is no AV concordance (nor VA concordance). Although the cardia is markedly malrotated and superiorly displaced there is no transposition anatomy. The mother and son ask if they could hear the results of the study, stating how the grave diagnosis had affected her, her husband’s, and her son’s life, and how they wondered each day if they would wake up to see their son dead, day after day, year after year, and how it affected his grades, his plans for the future, and his relationships with others including his ability to marry as he got older. Is good news bad here? Indeed, good news can be catastrophic because they both realized how very different the last 41 years might have been.
Case Study 7.
A 27-year-old black woman status post Jatene arterial switch for d-transposition of the great vessels referred for evaluation of right ventricle morphology and function (see Fig. 14-7). Instead of a Senning or Mustard atrial baffle or reconstruction technique, the patient underwent the arterial switch (Jatene) surgery. In the left panel, arrow points to the reinsertion of the coronary arteries as a button graft from the native pulmonary artery (PA), as well as the anterior positioned RV with its moderator band delineating the morphologic RV as the original systemic ventricle.
Case Study 8.
A 45-year-old woman with known ventricular septal defect (VSD) diagnosed at early childhood. After many years being lost to follow-up she is referred for CMR (see Fig. 14-8). Her examination no longer reveals a murmur of VSD but a harsh systolic ejection sound with loss of P2 and a prominent RV S4. Examination reveals hypertrophied RV free wall without VSD. In its place there is a prominent RV high septal muscle band creating dramatic subpulmonic stenosis (right panel) with a high velocity jet radiating into and across the pulmonic valve without frank pulmonic valvar stenosis. Spontaneous closure of VSDs has been shown to occur by several means, one of which is hypertrophy of the adjacent myocardial tissue, but on occasion is overzealous converting a volume overload situation into a pressure overload state, as in this case. The patient underwent myotomy with relief of the 98 mm Hg gradient and relief of symptoms.
Case Study 9.
Standard SSFP images performed in a 32-year-old woman referred for reasons unrelated to her final diagnosis. In Fig. 14-9 left panel, standard two-chamber view provides the rationale for why the large FOV is a valuable commodity for CMR, where a moderate-sized patent ductus arteriosum is seen. Using PVM, the Qp:Qs was quantified as 1.3:1 and she underwent uneventful percutaneous coil closure and went home the following day.
Case Study 10.
A 35-year-old man presents with a harsh pan-cyclic murmur, heard most prominently along his back, with symmetric pulse in his upper extremities but diminished pulses bilaterally in his lower extremities. A coarctation was suspected, as confirmed on the 20-second MRA acquisition (see Fig. 14-10, arrow). Note the severe coarctation and poststenotic dilatation. Additionally, an indication of the collateral circulation to the lower extremities can be gleaned from the widely dilated right internal mammary artery (chevron).
Case Study 11.
A 36-year-old man with a history of Tetralogy of Fallot and large left bronchial collaterals has undergone several repairs since age of 6 years. He presents with a 6-day history of chest pain and progressive dyspnea on exertion. His admitting electrocardiogram (ECG) reveals a right bundle branch block, and negative cardiac enzymes. The patient continued to deteriorate, requiring mechanical ventilation, whereupon a transesophageal echocardiography was performed which suggested an ascending aortic aneurysm with possible focal dissection and normal left and right sized ventricle. To further delineate the anatomy, a CMR was performed which revealed gargantuan left and right ventricles measuring 98 mm and 54 mm in enddiastole, respectively (see Fig. 14-11). The left ventricle was extensively compressed from the right ventricle, resulting in severe distortion in the superior-inferior direction. The left atrium was markedly dilated and tubular, due to marked posterior dilation and displacement of the ascending aortic aneurysm. The right ventricle was markedly dilated and hypertrophied with severe pulmonic, tricuspid, and AR. The sinus of Valsalva was markedly dilated measuring 61 mm whereas the proximal ascending aorta formed a gigantic aneurysm measuring 93 × 83 mm, terminating cranially into a nearly normal size aortic arch. Most importantly, there was a focal dissection in the anterior and right lateral wall of the aortic aneurysm. Additionally, several aorta-topulmonary (bronchial) collaterals were seen, with many communicating directly with the left atrium.