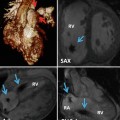
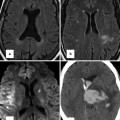
Fig. 29.1
Normal pericardium on CT (a, b) and MR (c, d) images. (b) Sagittal CT shows attachment of the fibrous pericardium to the central tendon of the diaphragm (dashed arrow). (a) CT and (c) MR axial images of the heart show the epicardial (inside) fat, the mediastinal (outside) fat, and the thin line of pericardial sac (arrows) between the two adipose tissue compartments. (d) Short-axis cine MR shows accumulation of fluid (Fl) in the pericardial sac separating the two fatty compartments. Parietal pericardium is shown by arrows in (d). In cine MR sequence (balanced steady-state free precession), it can be difficult to differentiate small amount of pericardial fluid from surrounding fat tissue as both appear high in signal intensity
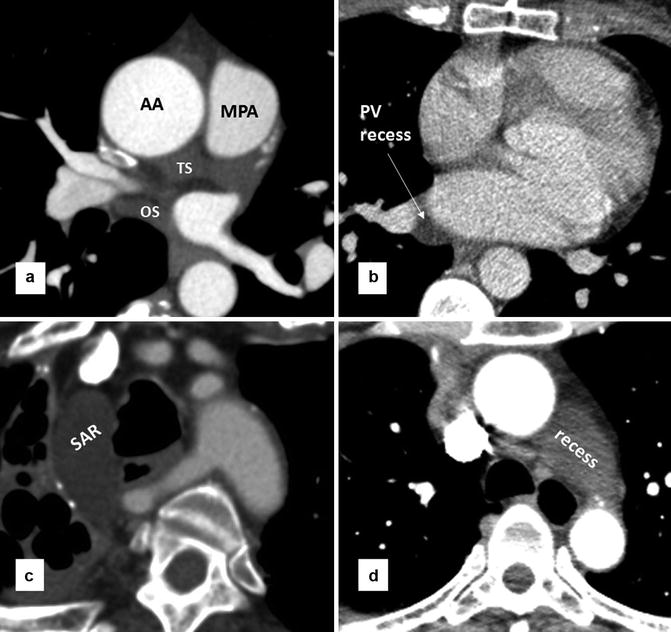
Fig. 29.2
Axial CT images of the heart show pericardial recesses. (a) Transverse sinus (TS) and oblique sinus (OS) are shown. (b) A prominent pericardial lateral recess of the pulmonary vein is shown. Lateral recesses are formed between superior and inferior pulmonary veins and commonly seen on the right side. (c) High riding aortocaval recess with its superior extent rising to the level of the thoracic inlet. (d) Large aortopulmonary pericardial recess. It is due to superior extension of the left pulmonic recess of the transverse sinus. AA ascending aorta, MPA main pulmonary artery, SAR superior aortic recess
The reflections of the serosal layers are arranged around two complex tubes. One tube encloses the aorta and pulmonary trunk. The second tube encloses the superior vena cava, the inferior vena cava, and the four pulmonary veins [2]. The transverse sinus is the passage between these two pericardial tubes and lies posterior to the ascending aorta and main pulmonary artery, above the left atrium (Fig. 29.2). The oblique sinus is the cul-de-sac located posterior and superior to the left atrium and separated from the transverse sinus by two layers of pericardial reflections that extend between the left and right superior pulmonary veins. The superior extent of the transverse sinus along the aortocaval space is the superior aortic recess [2]. Cephalad extension of this recess into the high right paratracheal region (high riding recess) can be mistaken for pathologies or a pericardial diverticulum [4, 5] (Fig. 29.2). Lateral extent of the transverse sinus forms the left and right pulmonic recesses between the pulmonary artery and superior pulmonary vein on each side [6]. Pericardial fat composes of the epicardial fat and the paracardial fat [7] (Fig. 29.1). Paracardial (mediastinal) fat is situated on the external surface of the parietal pericardium within the mediastinum. Epicardial (subepicardial) fat is located between the visceral layer of the pericardium and the myocardium. Epicardial fat is not limited to the surface of the heart but can penetrate deep in the interatrial septum and septal atrioventricular junction.
Imaging
CT and MRI are equally considered the preferred imaging methods for morphological assessment of the pericardial sac and the pericardium. Higher spatial resolution of the CT scan allows improve visualization of the normal pericardium. As a result normal pericardium appears thinner using CT compared with MRI. This is not surprising knowing that most cardiac CT protocols consist of 0.5–1 mm slices, while in cardiac MRI most routine sequences involve 5–8 mm slices. Generally a pericardium thicker than 3 mm is considered abnormal [8]. On the other hand, MRI is the modality of choice for functional evaluation of heart and to differentiate constrictive pericarditis from restrictive cardiomyopathy [9, 10].
Although evaluation of pericardium and pericardial fluid is possible on non-gated CT scans, ECG-gated methods are usually preferred particularly when detailed evaluation of the pathologies is required. For example, pericardial cysts can be characterized in most non-gated CT scans of the heart, while detection of partial absence of pericardium may be optimally performed using gated CT.
Morphological study of the pericardium with MRI can be best obtained with black-blood fast spin-echo (BB-FSA) sequence. Five millimeters axial, coronal, and sagittal images can be obtained to cover the entire pericardium. In this sequence, double inversion recovery (IR) pulses are used to null the blood signal. To remove high signal intensity of fat, a third IR pulse can be included in the pulse sequence, the so-called triple IR. It is important to know that BB-FSE sequence is not designed to be a T1-weighted sequence. As a matter of fact in most applications it is proton density or T2 weighted; as a result the fluid signal may appear bright not dark (Fig. 29.3). To make it T1 weighted, echo time should be lowered. T2-weighted triple IR sequence has the advantage of demonstration of pericardial edema in cases with inflammatory pericarditis. IR pulse can be adjusted to null pericardial fluid signal (Fig. 29.3). The second most important sequence in the evaluation anatomy as well as regional and global myocardial function with MRI is bright-blood balanced steady-state free precession (b-SSFP). This sequence is mainly T2 weighted and the signal of blood, fat, and pericardial fluid appears bright. Both non-ECG-gated and cine versions of b-SSFP are helpful for evaluation of the pericardium. Although advanced sequences such as tagged MRI and free-breathing real-time SSFP may be better choices for complete analysis of constrictive physiology and to study physiologic events such as ventricular coupling [9], by careful reviewing of routine cine images, it is simply possible to diagnose constrictive pericarditis in most cases (Fig. 29.4). Cine viewing allows better differentiation of motion between the rigid constricted pericardium and the underlying moving myocardium.
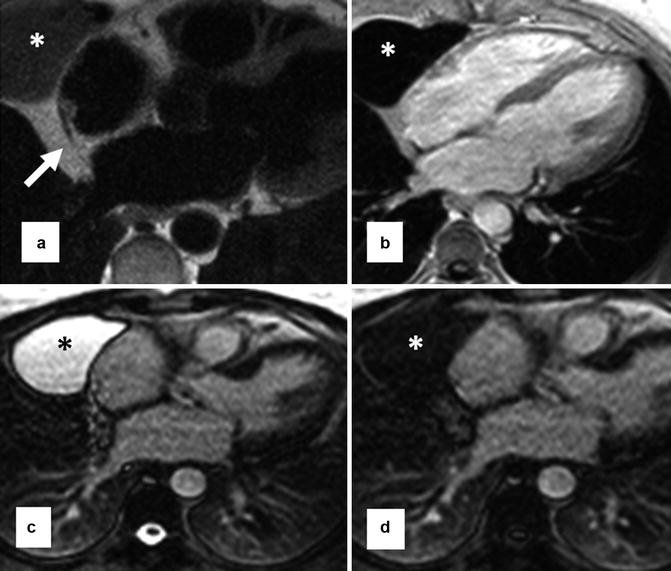
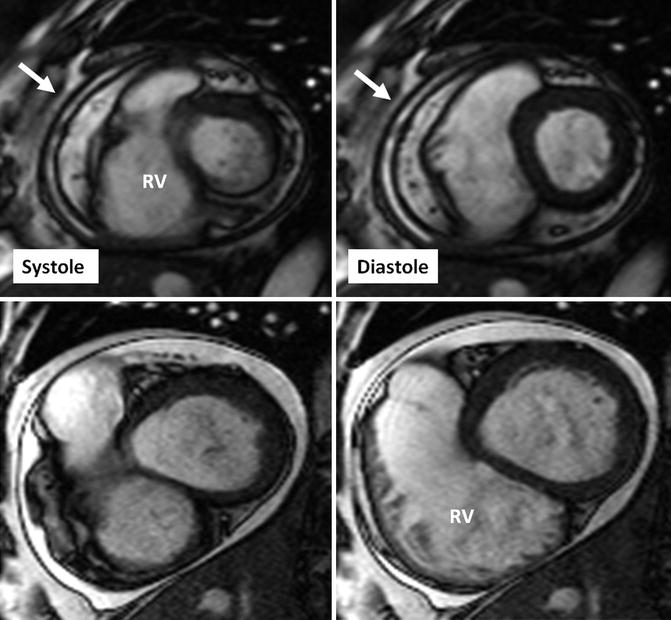

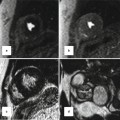
Fig. 29.3
Pericardial cyst (stars) in the right anterior cardiophrenic angle shown with MRI. (a) Black-blood fast spin echo shows the cyst as a homogeneous medium signal intensity area. This sequence is proton density weighted, and fluid-filled structures may appear isosignal to the myocardium. Note the cyst is separate from the normal pericardium (arrow). (b) Post-contrast T1-weighted fast gradient-echo sequence shows the cyst as a low-signal area. (c, d) are T2-weighted single-shot fast spin-echo sequences before (a) and after (b) fluid attenuation inversion pulse

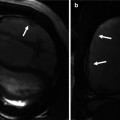
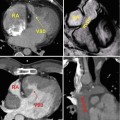
Fig. 29.4
Short-axis cine MR views in systole and diastole in a patient with constrictive pericarditis (upper row) and another patient with serous pericardial effusion. Images in constrictive pericarditis patient show thickened rigid pericardium with a fixed pericardial cavity (arrows) on both systolic and diastolic images. In contrast, the pericardium appears thin and smooth in pericardial effusion case (lower images), and the pericardial cavity (bright in signal intensity) covering the right ventricle (RV) appears smaller in diastolic image due to the shift of free fluid in the pericardial sac
Absence of Pericardial Tissue
The pericardial and pleural cavities and diaphragm are so closely related in their development.
The pericardial cavity represents a portion of the original right and left intraembryonic coeloms, and its lining derives from both the parietal (somatic) and the visceral (splanchnic) layers of the mesoderm. Later, the right and left side coeloms fuse, and folds of tissue divide it into separate cavities. The pericardial cavity separates from the peritoneal cavity by the formation of the septum transversum and from the pleura by the growth of the left and right pleuropericardial folds which are linked to the evolution of the incoming cardinal veins of the sinus venosus. Major part of pericardium is derived from the parietal mesoderm which is also primarily the wall of the pericardial cavity. A small dorsal portion is probably derived from the mesoderm at the root of the dorsal mesocardium. The septum transversum (a derivative of the visceral layer) forms the caudal wall of the pericardial cavity. Congenital pericardial defects result from incomplete development of either the septum transversum or the pleuropericardial folds. Premature atrophy of the left common cardinal vein (duct of Cuvier) compromises the blood supply to the left pleuropericardial fold and accounts for absence of the left pericardium [11, 12]. If abnormality occurs in early stages of embryonic development, the pleural serosa and diaphragm may also be affected.
Congenital absence of pericardium has been described in literature for more than four centuries and mainly discovered inadvertently during postmortem and thoracotomy procedures [11–14]. Radiologically, it was undiscovered until 1959 when Ellis et al. reported the first case by x-ray [15]. Subsequently, increasing numbers of cases have been reported. Van Son et al. presented the Mayo Clinic experience on congenital absence of the pericardium (15 cases in 34,000 surgeries) [16]. In all, the diagnosis was made in the operating room.
Congenital absence of pericardium occurs on a spectrum from complete absence of the left, right, or both sides of the pericardium to small regional defects. Partial defect may involve part of the left, right, or diaphragmatic pericardium. Approximately 30 % of patients with pericardial agenesis have additional anomalies such as atrial septal defect, bicuspid aortic valve, mitral stenosis, tricuspid insufficiency, patent ductus arteriosus, tetralogy of Fallot, pulmonary sequestration, bronchogenic cyst, and congenital diaphragmatic hernia [14–19]. Pectus excavatum is a common deformity in these patients [14] (Fig. 29.5). Agenesis of the left pericardium is the most common type accounting for 70 % of cases (Fig. 29.6). It usually involves the entire left side. In Gatzoulis series of ten cases with isolated pericardial defect, 70 % were complete absence of the left side [8]. Partial defects of left pericardium are not uncommon; they are usually located in the upper part of the left pericardium causing herniation of the left atrial appendage or part of the left ventricle (Fig. 29.7). In lower part is involved incarceration of the left ventricle and ischemia by coronary artery compression may ensue [20, 21]. Total aplasia of the pericardium accounts for less than 10 % of the cases identified [8, 16, 17]. It may coexist with an incomplete closure of the anterior chest wall, ectopia of the heart, and omphalocele. In less severe forms, the heart remains intrathoracic but falls over in the left hemithorax and may be associated with diaphragmatic defects. Agenesis of the right pleuropericardial membrane is considered very uncommon (4 %) (Fig. 29.8). Right-sided mediastinal shift of the heart may be observed. In the partial form, the right atrial appendage or ventricle may herniate through the defect or the right lung may herniate in and compress the superior vena cava [22–25]. Defects of the diaphragmatic pericardium are associated with partial diaphragmatic aplasia and intrapericardial herniation of the greater omentum.
Get Clinical Tree app for offline access

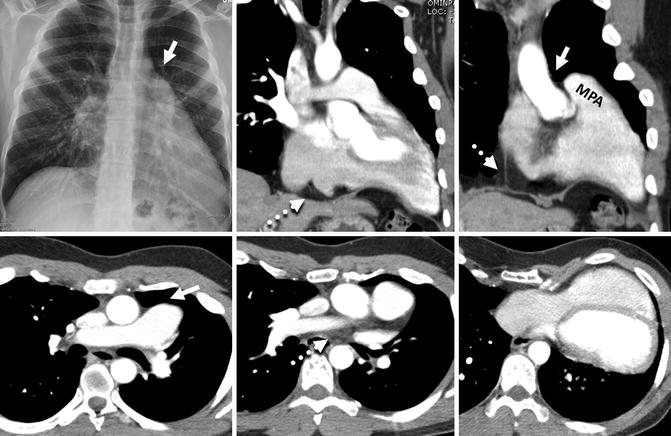
Fig. 29.5
Complete absence of the left pericardium in a 24-year-old male with pectus excavatum. The entire heart is shifted to the left. Plain radiograph and CT images demonstrate a prominent left main pulmonary artery extending beyond the mediastinal margins into the left lung. Because of the absence of left pericardium, a “tongue” of lung tissue interposes between the main pulmonary artery (MPA) and aorta (arrows), a diagnostic sign for congenital absence of pericardium. Only parts of the right pericardium extending to the diaphragm and left atrial appendage are seen (dashed arrows)

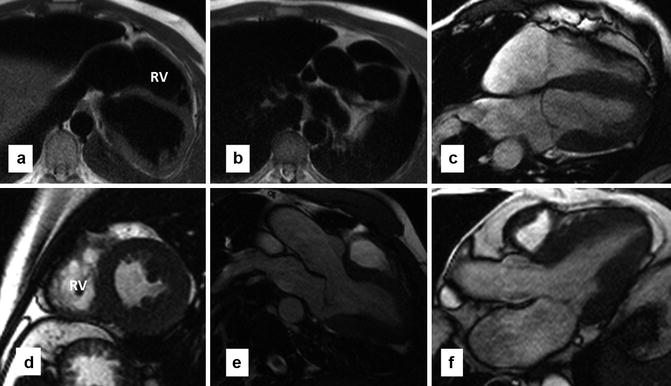
Fig. 29.6
MR images in a patient with complete absence of pericardium. (a, b) Are axial black-blood images. (c–e) Are cine images in four-chamber, axial, and three-chamber views, respectively. Normal pericardium cannot be traced. Dark line anterior to the right ventricle (RV) on short-axis view is related to susceptibility artifact not a real pericardial line. The heart is laterally displaced in the left hemithorax and the apex is rotated posteriorly. (f) Three-chamber view of a normal heart is shown for comparison

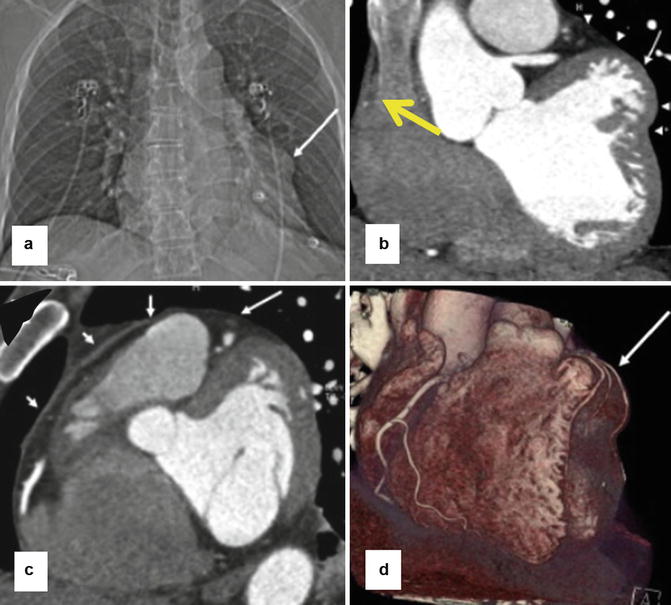
Fig. 29.7
Partial absence of the left pericardium in a 63-year-old man with severe chest pain that worsened with breathing. (a) CT topogram shows an abnormal bulge along left cardiac boarder (arrow). (b–d) CT coronary angiograms show that the focal bulge (long white arrows) is due to partially herniated left ventricle. Arrowheads in panel b




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree