Overview of the Uterine Anomalies
Unicornuate Uterus
The unicornuate uterus arises due to agenesis or hypoplasia of one of the two Müllerian ducts. The unicornuate uterus is a functional uterus with a normal-appearing cervix and a single fallopian tube, and the contralateral side may have a variety of configurations: agenesis or a rudimentary horn in 74 % [6]. The rudimentary horn can be noncommunicating (70 to 90 %) or communicating with the unicornuate uterus and may contain functional endometrium [20]. Women with a rudimentary uterine horn containing functional endometrium may present with cyclic or chronic pain, endometriosis, or a horn gestation [20]. Nonfunctional rudimentary horns are usually asymptomatic. Lastly, the unicornuate uterus is associated with a 40 % incidence of renal anomalies, usually ipsilateral to the anomalous side [20–22].
Uterus Didelphys
The uterus didelphys results from complete failure of lateral fusion of the two Müllerian ducts; duplication of the Müllerian structures is the result. Anatomically, these women have two unicornuate uteri, two separate endometrial cavities, and two cervices. In the majority of women with a uterus didelphys, vaginal duplication also occurs and a longitudinal vaginal septum is present. Additionally, this anomaly can present with an obstructed hemivagina and associated ipsilateral renal anomaly [23, 24].
Bicornuate Uterus
Incomplete lateral fusion of the Müllerian ducts at the fundus results in a bicornuate uterus. Commonly, a single cervix and two endometrial cavities are present. Variability exists in the extent of separation between the two cavities, with maximal separation extending down to the internal cervical os (complete bicornuate). A fundal indentation of at least 1 cm has been found to be reliable for differentiating a bicornuate from a septate uterus [11, 25–28]. Although a normal vagina is commonly present, a longitudinal vaginal septum can occur with the bicornuate uterus [14].
Septate Uterus
The septate uterus occurs due to a defect in resorption of the midline division between the two fused Müllerian ducts, and a fibromuscular septum remains. The degree of septation can vary from complete, extending from the uterine fundus through the cervix, to partial, in which a portion of the caudal aspect of the septum is resorbed. Since the Müllerian ducts are completely fused, a normal external fundal contour is present despite a complete or partial division of the endometrial cavity. A longitudinal vaginal septum is a common finding with a complete septate uterus and can also occur with a partial septate uterus [14]. Endometriosis is also associated with septate uteri and has been documented in 30 % of fertile and infertile women with septate uteri [29, 30].
Arcuate Uterus
The arcuate uterus demonstrates a slight, rounded midline septum with a broad fundus and sometimes has a small indentation at the fundus. It has been characterized as a variant of normal uterine anatomy or a uterus with a small partial septum [16], or a bicornuate uterus. Appropriate imaging to define uterine anatomy is essential so as not to misclassify a uterus as arcuate instead of partial septate or bicornuate, which have different reproductive implications.
Müllerian Agenesis
The most extreme of the Müllerian anomalies is Müllerian agenesis, otherwise known as Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome, which occurs due to agenesis or hypoplasia of the Müllerian ducts and affects approximately 1 in 5,000 females [31]. Müllerian agenesis involves congenital absence of the vagina and variable uterine development that ranges from agenesis to hypoplastic and rudimentary structures. One study demonstrated that in females with MRKH, 87 % had Müllerian remnants, 26 % of the remnants were cavitated and contained endometrial mucosa, 7 % had a Müllerian remnant measuring >4 cm, and 30 % had anomalies of the urinary tract [32]. Along with urologic anomalies, Müllerian agenesis is associated with other extragenital anomalies involving skeletal, cardiac, and auditory systems and digits and palate [33, 34].
Clinical Presentation of Congenital Uterine Anomalies
Although many females with congenital uterine anomalies are asymptomatic and a late diagnosis may occur during evaluation of infertility [35, 36], it is important to recognize several gynecologic and obstetric signs and symptoms that may indicate a uterine disorder (Table 9.1). Müllerian agenesis presents with primary amenorrhea. Women with an obstructive anomaly may report cyclic or noncyclic pelvic pain and dysmenorrhea, and these symptoms can begin several months after menarche or into adulthood. Obstructive uterine anomalies are associated with hematometra, retrograde menstruation, and endometriosis [21, 37]. Endometriosis is a common finding in women with obstructive and nonobstructive Müllerian anomalies and is a known etiology of infertility [29, 37]. Abnormal bleeding can also occur with uterine anomalies and has been associated with septate uteri [29]. Furthermore, vaginal anomalies may occur in conjunction with uterine anomalies, and abnormal bleeding may be due to a partial or microperforate vaginal obstruction or a longitudinal vaginal septum. A nonobstructive vaginal anomaly such as a longitudinal vaginal septum, which is associated with septate and didelphys uteri, may be a woman’s first presentation with a uterine anomaly; associated symptoms include difficulty with tampon insertion, bleeding around one tampon (two are required), and dyspareunia. Hence, if a vaginal anomaly is identified, then uterine imaging is warranted [14].
Table 9.1
Clinical presentation of uterine anomalies
Gynecology | Obstetrics |
|---|---|
Pelvic pain, cyclic or noncyclic | Pregnancy loss: first and second trimester |
Dysmenorrhea | Cervical incompetence |
Primary amenorrhea with pain | Preterm labor and delivery |
Primary amenorrhea without pain | Intrauterine growth restriction |
Hematometra | Placental abruption |
Abnormal uterine bleeding | Intrauterine fetal demise |
Dyspareunia | Malpresentation |
Cesarean delivery | |
Pregnancy-induced hypertension (related to renal abnormalities) | |
Pregnancy in rudimentary uterine horn |
In obstetrics, congenital uterine anomalies are associated with a higher rate of poor obstetric outcomes: recurrent pregnancy loss (RPL), first and second trimester pregnancy loss, intrauterine growth restriction, preterm labor and delivery, placental abruption, malpresentation, and intrauterine fetal demise [1, 7, 21, 38, 39]. Among women with RPL, the incidence of uterine anomalies is highly variable and ranges from 6 to 38 %, but based on meta-analyses is likely closer to 12 to 16 % and is as high as 25 % in women with second trimester pregnancy loss [3–5, 40]. Uterine dysfunction may occur due to diminished cavity size, insufficient musculature, impaired ability to distend, abnormal myometrial and cervical function, inadequate vascularity, or abnormal endometrial development [1, 3, 8, 22, 41–46]. Due to higher rates of malpresentation, an increased rate of cesarean delivery can be seen with uterine anomalies. Additional obstetric complications such as cervical incompetence [47], pregnancy-induced hypertension (due to renal anomalies), and antepartum and postpartum bleeding are also associated with congenital uterine anomalies. Lastly, pregnancy may occur in an obstructed or rudimentary uterine horn. These pregnancies are surgical emergencies due to an 89 % rate of rupture and the related morbidity and mortality [20].
Imaging of Congenital Uterine Anomalies
Initial testing to evaluate pelvic anatomy, especially in infertile women, may include hysterosalpingography (HSG) and two-dimensional ultrasonography (2DUS). While these modalities are useful for the initial assessment of uterine anomalies, additional testing may be warranted such as saline-infusion ultrasonography (SIS), magnetic resonance imaging (MRI), and the increasingly common technique of three-dimensional ultrasonography (3DUS). The benefit of 3DUS and MRI is the ability to simultaneously assess the uterine fundus and cavity [17]. However, there are inherent strengths and limitations to each imaging technique; thus, a combination of several techniques may be necessary to evaluate a uterine anomaly. Although surgical evaluation (i.e., laparoscopy, hysteroscopy, laparotomy) has been considered the gold standard for evaluation of complex Müllerian anomalies [18, 41], with readily available diagnostic imaging, surgery is infrequently necessary for evaluation and diagnosis of anomalies. Surgical intervention with hysteroscopy and/or laparoscopy may only be necessary when the uterine anomaly is amenable to surgery and the intervention is clinically necessary [4, 48, 49]. This discussion will review all available imaging techniques and will focus on the evolving technique of 3-D ultrasonography.
Hysterosalpingography
A common procedure for evaluation of tubal patency in women with infertility, HSG can also provide information about the contour of the uterine cavity. In a woman with a uterine anomaly, the HSG may identify patent canals and any complex communications, but is unable to adequately evaluate the external uterine contour and, hence, cannot reliably differentiate between uterine anomalies [4, 11, 35]. When a uterine anomaly is identified, assessment of the external uterine contour can be achieved with 2DUS, 3DUS, and/or SIS. In one study, HSG correctly diagnosed 55 % of septate and bicornuate uteri, and the addition of ultrasonography improved this result to 90 % [50]. Since the HSG involves exposure to ionizing radiation, in young women with desired fertility, this test should only be ordered when clinically indicated.
Two-Dimensional Ultrasonography
Two-dimensional transabdominal or transvaginal ultrasonography is a common initial technique for assessing pelvic structures. It effectively visualizes the uterine structure and endometrial contour, can detect a pelvic mass or hematometra, confirms the presence of ovaries, and can be used to evaluate the kidneys. When 2DUS is performed in the secretory phase of the menstrual cycle, better visualization of the endometrium and internal uterine contour can be achieved [51, 52]. A compilation of 2DUS studies for uterine anomalies noted a pattern of low sensitivity and high specificity; although 2DUS can only identify about half of the uterine anomalies present, the diagnosis of an anomaly is highly likely to be correct [4]. When indicated, saline infusion sonography can be employed to further assess the internal and external uterine contours and can accurately diagnose uterine anomalies as well as identify other intracavitary abnormalities such as polyps, myomas, or adhesions [4, 49, 53].
Pelvic Magnetic Resonance Imaging
Pelvic MRI is a sensitive and specific imaging modality for evaluating Müllerian anomalies [11, 54]. MRI provides detailed delineation of internal and external uterine contours, can differentiate between a myometrial and fibrous uterine division, can differentiate between a septate cervix and duplicated cervix, can diagnose vaginal anomalies, and can identify if a rudimentary uterine horn contains functional endometrium [11, 17]. Furthermore, MRI can also assess renal morphology and location. Although costly, this noninvasive imaging modality is less expensive than surgery [18]. Pelvic MRI may not be necessary for every patient with a uterine anomaly and may be best utilized for the evaluation of complex Müllerian anomalies [17, 36].
A number of studies have evaluated the efficacy of MRI to assess surgically confirmed uterine anomalies [18, 55–58]. A range of sensitivity (29 to 100 %) and specificity (33 to 100 %) and positive predictive value (83 to 100 %) and negative predictive value (25 to 100 %) was identified. The ability of MRI to detect and correctly diagnose a uterine anomaly can be limited by the availability of technically adequate images which may be influenced by the MRI machine and software utilized and requires image interpretation by a practitioner with experience in the diagnosis of uterine anomalies [18, 49].
Three-Dimensional Ultrasonography
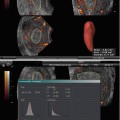
Three-dimensional ultrasonography (3DUS) is a relatively new imaging technique that provides detailed and highly accurate views of pelvic anatomy; it constructs three-dimensional volumes from a series of two-dimensional images [18, 27]. After the volume is created, it can be stored and any section of a structure can be examined. With uterine anomalies, the ability to visualize the coronal section of the uterus is invaluable for assessing the architecture of the endometrial cavity and the uterine fundus (Fig. 9.2) [17, 27, 48, 59, 60]. Therefore, by evaluating the internal and external uterine contours, 3DUS is able to reliably differentiate between various uterine anomalies and can assess the often subtle differences between septate and bicornuate uteri [17, 18, 27, 28, 59, 61]. However, distortion by leiomyomas may make uterine assessment more challenging [7, 18, 59]. This modality is less expensive and less time consuming than surgery or pelvic MRI, is less invasive than surgery, and may be better tolerated [17, 18, 52]. Although the American Fertility Society classification for uterine anomalies (Fig. 9.1) does not provide dimensions or measurements to enable differentiation of uterine anomalies based on ultrasound findings, a modification of the AFS criteria based on 3DUS landmarks has been utilized to facilitate the diagnosis of uterine anomalies (Table 9.2, Fig. 9.3) [11, 16, 18, 19, 48, 61].
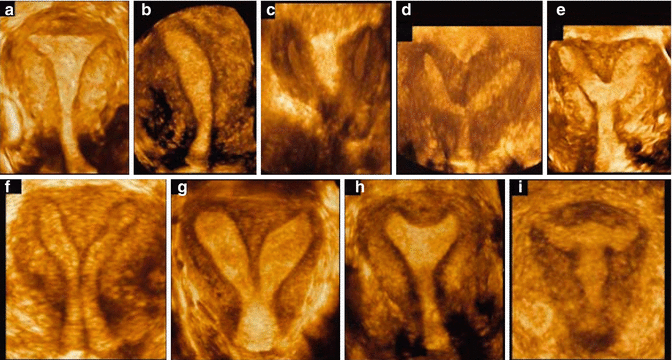
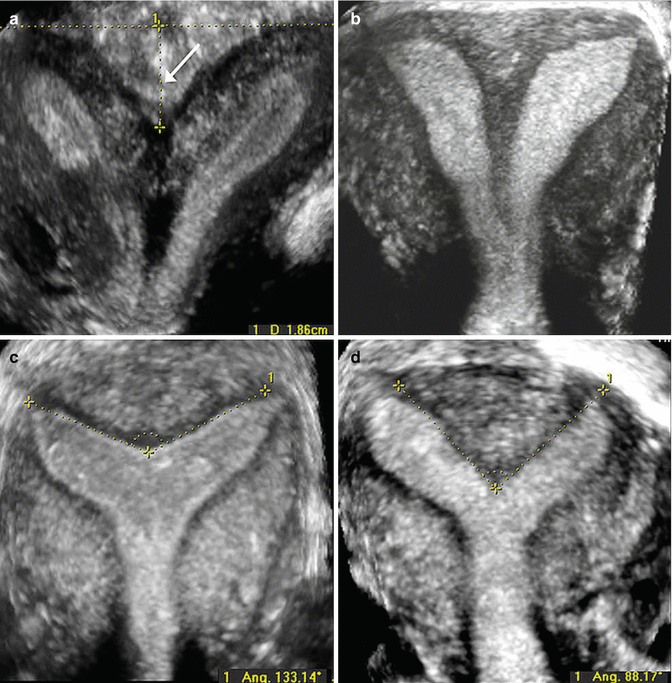

Fig. 9.2
Three-dimensional rendered coronal ultrasound images demonstrating different uterine anomalies using the American Fertility Society classification [16]: (a) normal uterus; (b) unicornuate uterus; (c) didelphic uterus; (d) complete bicornuate uterus; (e) partial bicornuate uterus; (f) complete septate uterus; (g) partial septate uterus; (h) arcuate uterus; (i) uterus with diethylstilbestrol (DES) drug-related malformations (Reprinted Bermejo et al. [17]. With permission from John Wiley & Sons, Inc.)
Table 9.2
Three-dimensional ultrasound criteria for classification of congenital uterine anomalies
Uterine morphology | Fundal contour | External contour |
|---|---|---|
Normal | Straight or convex | Uniformly convex or with indentation <10 mm |
Arcuate | Concave fundal indentation with central point of indentation at obtuse angle (>90°) | Uniformly convex or with indentation <10 mm |
Partial septate | Presence of septum (does not extend to cervix) with central point of septum at an acute angle (<90 %) | Uniformly convex or with indentation <10 mm |
Complete septate | Presence of septum that completely divides cavity from fundus to cervix | Uniformly convex or with indentation <10 mm |
Bicornuate | Two well-formed uterine cornua | Fundal indentation >10 mm dividing the two cornua |
Unicornuate uterus | Single well-formed uterine cavity with a single interstitial portion of fallopian tube and concave fundal contour | Fundal indentation >10 mm dividing the two cornua if a rudimentary horn is present |

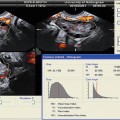
Fig. 9.3
Three-dimensional rendered coronal ultrasound images demonstrating ultrasound criteria for classification of congenital uterine anomalies. (a) Bicornuate uterus: two divergent cornua are noted, divided by a sagittal cleft >10 mm (arrow). (b) Complete septate uterus: a normal external uterine contour is present, and a septum divides the endometrial cavity and extends to the cervix. (c) Arcuate uterus: a normal external uterine contour is identified with a concave fundal indentation of the endometrial cavity at an obtuse angle. (d) Partial septate uterus: a normal external uterine contour is present, the septum does not extend to the cervix, and the central point of the fundal indentation demonstrates an acute angle (Reprinted Ghi et al. [48]. With permission from Elsevier)
When compared to HSG and 2DUS, 3DUS demonstrates high sensitivity and specificity for the identification of a normal uterus (98 and 100 %), arcuate uterus (100 and 100 %), or major uterine anomaly (100 and 100 %) [59]. In comparison, 2DUS has lower sensitivity and specificity for the diagnosis of a normal uterus (88 and 94 %) or arcuate uterus (67 and 94 %), but is similarly accurate with major uterine anomalies (100 and 95 %). Hence, 2DUS may be best utilized as a screening test for uterine anomalies, with 3DUS as the definitive diagnostic test [59].
Several studies investigated the accuracy of 3DUS for the evaluation and diagnosis of uterine anomalies and confirmed the radiologic findings at surgery (laparoscopy and/or hysteroscopy). In one study, 3DUS assessment of the uterine fundus correlated 91.6 % with laparoscopic findings, and evaluation of the uterine cavity correlated 100 % with hysterosalpingography [62]. Wu et al. compared 3DUS with laparoscopy for the detection of uterine anomalies, and 3DUS demonstrated 100 % sensitivity and specificity and correctly diagnosed 92 % (11/12) of septate uteri and 100 % (3/3) of bicornuate uteri [28]. A study of 3,850 infertile women who underwent uterine evaluation with 3DUS and hysteroscopy identified 689 (17.9 %) with septate uteri, and 3DUS demonstrated 99.27 % sensitivity and 100 % specificity for diagnosing a septate uterus [7]. Another recent study investigated 254 nulliparous women with recurrent pregnancy loss, and 3DUS findings were confirmed by office hysteroscopy (for normal uteri) or laparoscopy/hysteroscopy if a uterine anomaly was identified [48]. Fifty-four subjects (19 %) were diagnosed with a uterine anomaly, and 3DUS correctly identified 52 (92.3 %) of the anomalies; two partial septate uteri were misclassified as bicornuate and arcuate. When 3DUS and 2DUS were compared for the diagnosis of uterine anomalies during different phases of the menstrual cycle, both modalities had higher sensitivity and specificity during the luteal phase, but 3DUS demonstrated greater sensitivity and specificity in both the follicular and luteal phases, and the diagnostic accuracy of 3DUS was comparable to HSG, hysteroscopy, and laparoscopy [52]. Lastly, the reproducibility of the interpretation of 3DUS volumes to diagnose uterine anomalies has been established [61].
Few studies have compared the diagnosis of uterine anomalies by 3DUS versus pelvic MRI. Bermejo et al. determined that in women with uterine anomalies, 3DUS and MRI demonstrate a high degree of concordance, with a kappa index of 0.880 (95 % CI, 0.77 to 0.99) [17]. Discrepancies occurred in the diagnosis of 4 of 65 anomalies; 3DUS misclassified one bicornuate uterus as uterus didelphys, and 3 septate uteri as bicornuate uteri. In contrast, Faivre et al. investigated women with suspected septate and bicornuate uteri; all 31 uterine anomalies were confirmed by hysteroscopy and/or laparoscopy [49]. 3DUS correctly identified 31/31 uterine anomalies, and pelvic MRI correctly identified 24/31 uterine anomalies; five septate uteri were misclassified as bicornuate uteri, and 2 partial septate uteri as complete septate uteri. These discrepancies were attributed to the lack of a coronal uterine image and lack of familiarity with the evaluation of uterine anomalies.
3DUS has been demonstrated to be at least as accurate as pelvic MRI for diagnosing uterine anomalies. However, 3DUS is not a widely available imaging modality and requires a high level of practitioner skill and experience to achieve high diagnostic accuracy [17, 18, 57]. Although these studies have promising results, it must be emphasized that they were performed by practitioners with expertise in the performance and interpretation of 3DUS and in the diagnosis of uterine anomalies.
Urinary Tract Imaging
Lastly, since urinary tract anomalies are associated with Müllerian anomalies, imaging of the urinary tract needs to be considered when a uterine anomaly is identified. Upper urinary tract anomalies include renal agenesis, horseshoe or pelvic kidney, duplication of the collecting system, or an ectopic ureter [10]. Renal anomalies most commonly occur with unicornuate and didelphic uteri and with Müllerian agenesis and are infrequently identified with bicornuate, septate, and arcuate uteri [63]. If an obstructive Müllerian anomaly is identified such as a unicornuate uterus with a rudimentary uterine horn or uterus didelphys with an obstructed hemivagina, renal anomalies including renal agenesis are commonly identified ipsilateral to the obstruction. In more than 50 % of cases, renal agenesis is predictive of an obstructive Müllerian anomaly [20].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree