1 Conventional Radiography In cardiology as in other specialties, the indication for examining the heart is based on patient complaints.1–8 In this section the main clinical manifestations of heart diseases are briefly reviewed from a differential diagnostic standpoint. Conventional X-ray films of the chest, or projection radiographs, are a basic tool in the differential diagnostic investigation of these symptom complexes.8 This complaint is almost always related to heart disease. It typically induces fear of an imminent life-threatening heart attack. If the pain is of brief duration, it is referred to as a typical episode of angina pectoris. Approximately one-third of patients with myocardial ischemia, especially diabetics, do not experience chest pain. If the pain lasts longer than 15 minutes, extracardiac causes should also be considered. The differential diagnosis of chest pain is thus highly complex: • Angina pectoris (myocardial ischemia), myocardial infarction • Hypertrophic cardiomyopathy • Dilated cardiomyopathy • Arrhythmias • Acute pericarditis (pericarditis sicca) • Mitral valve prolapse • Aortic aneurysm • Aortic dissection • Massive pulmonary embolism • Small or moderate pulmonary embolism combined with pulmonary infarction • Pneumothorax • Pleuritis sicca • Pneumonia • Broad range of esophageal disorders • Broad range of musculoskeletal disorders • Broad range of abdominal diseases in which pain is projected to the chest • Neurogenic diseases (neuralgia, radicular syndromes, herpes zoster) • Functional cardiac complaints The differential diagnosis of chest pain is extremely challenging. The establishment of a chest pain unit in the emergency room, chiefly in university hospitals, is an initiative aimed at addressing this problem. Edema results from an imbalance between the quantity of fluids, salts, and proteins which leave and re-enter the capillary system. It may be a manifestation of heart disease. A classic example is biventricular heart failure, which may develop as a result of myocardial, pericardial, or valvular heart disease, as well as of other cardiac lesions. Dyspnea, or respiratory distress, is characterized by very different degrees of severity; it may occur during rest or exercise, and may be position-dependent (orthopnea) or may present as an abnormal respiratory pattern. The underlying cause may be a disturbance of ventilation, gas exchange, or lung perfusion. In addition to pulmonary causes, various cardiac abnormalities may be the underlying symptoms of respiratory distress. Possible causes include pulmonary outflow obstruction or left-sided heart failure as a result of myocardial, valvular, or coronary heart disease. Cyanosis is a reflection of insufficient pulmonary oxygen uptake or peripheral oxygen depletion. Pulmonary cyanosis may be caused by: • Ventilatory impairment • Diffusion impairment • Increased intrapulmonary venous admixture of blood in unventilated but perfused lung areas, or the arteriovenous shunting of blood. Cardiac central cyanosis may be caused by: • Congenital heart defects with a right-to-left shunt • Congenital defects with decreased pulmonary blood flow These heart defects lead to central cyanosis. This condition is to be distinguished from peripheral cardiac cyanosis, in which cardiac output is reduced by myocardial insufficiency or caused by congestion, e. g., mitral stenosis). Tachycardiac arrhythmias are present when the rate of ventricular contractions increases to 100 bpm or more. They may occur in association with: • Primary defects of the impulse conduction system (e. g., congenital anomalies) • Coronary heart disease • Myocardial diseases • Valvular heart diseases • Severe left ventricular dysfunction • Pericardial diseases • Right ventricular dysplasia (cardiomyopathy) The potential causes of hypertension are extremely diverse. The most important causes are listed below: • Decreased elasticity of the aorta and great vessels due to atherosclerosis • Coarctation of the aorta • Conditions causing an increase in stroke volume and cardiac output • Heart failure • Renal causes Over time, all of these conditions lead to cardiac symptoms which may be reflected in a change in the cardiac silhouette. Primary forms of cardiac hypotension result from a decrease in cardiac output due to • Impaired myocardial contractility (heart failure) • Cardiac arrhythmias (bradycardiac and tachycardiac) • Impaired diastolic filling of the heart • Valvular heart disease The most common form of acute cardiac hypotension is cardiogenic shock due to myocardial infarction or severe heart failure. Chronic cardiovascular forms of hypotension are associated with the following conditions: • Aortic valve disease • Mitral valve disease • Aortic arch syndrome • Constrictive pericarditis Brief syncopal episodes lasting 1 minute or less may have a cardiac cause. The cause may be mechanical, relating to abnormalities of left ventricular emptying or filling. Syncope may also result from septal defects (including atrial septal defects), as well as thrombus formation in the left atrium associated with mitral stenosis. The following mechanical causes of syncope can be differentiated by imaging studies: • Abnormalities of left ventricular emptying • Abnormalities of left atrial emptying • Aortic stenosis • Hypertrophic obstructive cardiomyopathy • Heart failure • Myocardial infarction Conventional radiography continues to have an established place even with the availability of modern electrophysiology, echocardiography, invasive cardiac testing, and sectional imaging modalities such as CT and MRI, although its role needs to be redefined.9–18 Thus, sophisticated cardiological tests are not state of the art unless standard biplane chest films are also obtained. Radiographs are also widely used in the follow-up of cardiac status. This applies to the follow-up of conservative and interventional therapies and, in particular, to preoperative treatment planning or perioperative monitoring and follow-up after surgical intervention.6,19–21 The information necessary for understanding and interpreting a normal chest radiograph is derived from specific pathophysiological parameters that are based on changes in hemodynamics. The standard projections for cardiac radiography are the frontal (posteroanterior, P-A) projection and the left lateral projection (with an esophagogram). A plain radiograph of the heart is a summation image of the individual cardiac chambers. The cardiac borders and their shape changes are therefore the sole indicators in determining the location of the individual cardiac chambers and assessing their size in radiographs. The structures that form the borders of the heart cannot always be conclusively identified, even when all standard views are obtained (Fig. 1.1).22 The following structures normally form the cardiac borders in the frontal radiograph: • Left side of mediastinum (from above downward): – Distal portion of the aortic arch – Main pulmonary artery trunk – Left atrial appendage (part of the left atrium) – Left ventricle • Right side of mediastinum (from above downward): – Superior vena cava – Right atrium The protrusion located below the aortic arch on the P-A radiograph is termed the pulmonary segment. This is an arch-shaped structure formed by the pulmonary trunk and not by the conus pulmonalis, which is located below the pulmonary valves. The pulmonary segment may be very prominent owing to dilatation of the pulmonary artery. The lateral radiograph shows the following topography: • Anterior structures (from above downward): – Ascending aorta – Pulmonary trunk – Right ventricle Fig. 1.1a, b Diagrammatic representation of the radiographic anatomy of the heart. a P-A radiograph. b Lateral radiograph. The retrosternal space normally appears as a narrow space between the anterosuperior border of the heart and the anterior chest wall. • Posterior structures (from above downward): – Descending aorta and pulmonary vessels – Left atrium – Left ventricle – Inferior vena cava The space between the posterior cardiac border and vertebral column represents the retrocardiac space. The space bounded by the left atrium, vertebral column, and aortic arch (superior vascular pedicle) is called the aortic window. If the aortic window is notably devoid of structures, this indicates the presence of small main pulmonary arteries. The close relationship between the esophagus and left atrium explains why an enlarged left atrium causes circumscribed posterior displacement of the esophagus in the lateral chest radiograph. Given the typical location and arrangement of the cardiac chambers, the enlargement of specific chambers may produce characteristic changes in the size and shape of the cardiac silhouette that can be identified in the various projections.23–25 Conventional radiographic examination of the heart begins with a P-A projection, which is obtained when the patient is first admitted. A left lateral radiograph at full inspiration shows an overview of the anterior and posterior cardiac borders and may yield important information on the relative sizes of the cardiac chambers.26 Today, the traditional right anterior oblique (RAO) and left anterior oblique (LAO) views of the chest are important only in angiocardiography. They are no longer included in a standard radiographic series. Oral contrast radiography of the esophagus, especially in the lateral projection, is useful for evaluating the position of the aortic arch, the course of the descending aorta, and anomalies of the aortic arch vessels. It is also useful in the assessment of the size of the left atrium on the posterior border of the heart. Cardiac fluoroscopy, in which different projections are obtained by rotating the patient, is used to screen for cardiac calcifications and to evaluate the motion of the cardiac borders. Despite its demonstrable value, fluoroscopy is rarely included in routine cardiac imaging. It can supply information on: • Severely calcified coronary vessels • Calcified cardiac valves • Calcifications of the myocardium, pericardium, or fibrous ring • Movement and location of implanted pacemaker leads • Wobbling or other instability of implanted valves or valve rings (Fig. 1.2) P-A View When the right ventricle is enlarged, it expands upward in the direction of its outflow tract and from right (posterior) to left (anterior), and to the side (Fig. 1.3). This displaces the pulmonary artery upward, causing it to occupy most of the concavity of the cardiac waist in the P-A view. This type of cardiac silhouette is referred to as a right ventricular configuration. It is a common normal finding in children and is very infrequently found in healthy adolescents and adults. At the same time, the associated rotation of the heart toward the left side shifts the left ventricle more or even completely toward the posterior side of the heart. The right ventricle may expand on the anterior side of the heart and, in rare cases, forms the left border of the cardiac silhouette (Fig. 1.4). Fig. 1.2a–f Sites of occurrence of intracardiac calcifications (diagrammatic representation). a Calcified mitral valve annulus; P-A and lateral views. b Calcification of the aortic and mitral valve; lateral view. c Calcification of the LAD and left coronary artery; RAO view. d Calcification of the left atrial wall; P-A and lateral views. e Calcification of the pericardium, calcifying pericarditis; P-A and RAO views. f Calcified myocardial aneurysm; P-A view. Dilatation of the right ventricle rotates the heart to the left, shifting the left ventricle posteriorly about the cardiac axis. Lateral View In the left lateral view, dilatation of the right ventricle leads to narrowing and obliteration of the retrosternal space. This is considered a reliable sign of right ventricular enlargement. When enlargement is extreme, the left ventricle is shifted so far posteriorly that it may cause narrowing of the retrocardiac space near the diaphragm (Fig. 1.5). P-A View Enlargement of the left ventricle expands the heart downward, backward, and toward the left side. The cardiac silhouette in the P-A view appears broadened, and the cardiac waist is accentuated. This pattern is generally described as an aortic or left ventricular configuration. With few exceptions (congenital heart defects, e. g., Fallot-type cardiac anomalies), it is typical of left ventricular enlargement (Figs. 1.6, 1.7). Fig. 1.3a, b Change in cardiac shape caused by pronounced enlargement of the right ventricle. a P-A radiograph. b Lateral radiograph. Fig. 1.4 Diagrammatic representation of the change in cardiac shape caused by significant rotation to the right (left ventricular dilatation) and to the left (right ventricular dilatation) compared with the normal position. Fig. 1.5a–c Pronounced right ventricular configuration in stage III cor pulmonale. With the heart rotated to the left, the dilated right ventricle forms the anterior cardiac border. It also extends to the left cardiac border, displacing the left ventricle posteriorly (c). Typical signs of pulmonary hypertension are: • Prominence of the pulmonary segment • Large central pulmonary arteries • Cardiac rotation due to right ventricular dilatation. a P-A radiograph. b Left lateral radiograph with esophagogram. c Cardiac CT scan at the level of the ventricular plane demonstrates the dilated right ventricle, the nearly horizontal orientation of the septum, and posterior displacement of the left ventricle. The P-A view is useful in distinguishing between dilatation of the right and left ventricles, as it enables one to differentiate between a right ventricular configuration of the cardiac silhouette and an aortic or left ventricular configuration. Lateral View Narrowing of the retrocardiac space is an indicator of left ventricular enlargement in the left lateral radiograph. The left ventricle is definitely enlarged if it extends more than 18 mm past the inferior vena cava. The enlarged left ventricle may displace the lower portion of the barium-filled esophagus, forming a posterior convexity, or it may slide back past the esophagus without altering its course. P-A View Normally the right atrium forms almost the entire right border of the cardiac silhouette in the P-A view. An enlarged right atrium forms a convex protrusion toward the right, which may be most conspicuous at the upper right cardiac border (Fig. 1.8). The enlarged right atrium also expands toward the left side, however, and broadening of the heart on the right side is thus not directly proportional to the degree of atrial dilatation. Fig. 1.6a, b Change in cardiac shape caused by left ventricular enlargement in the P-A radiograph (a) and lateral radiograph (b). Fig. 1.7 Typical aortic or left ventricular configuration in a patient with combined aortic valve disease with left ventricular dilatation. The ascending aorta is expanded because of a jet effect resulting from aortic stenosis. Fig. 1.8 Change in cardiac shape caused by dilatation of the right atrium. When the left atrium is enlarged, it expands posteriorly in accordance with its location on the posterior heart wall. It may also project toward the right side, and less commonly it may broaden the left side of the cardiac silhouette. Because the left atrium is located in close proximity to the esophagus, dilatation of the left atrium always leads to circumscribed displacement of the barium-filled esophagus. The size of the left atrium can be determined on an upright contrast radiograph of the esophagus in the left lateral projection taken at full inspiration. When the dilated left atrium is viewed in the frontal chest radiograph, the superimposed positions of the left and right atria often create a double contour along the upper right cardiac border, especially taken with harder x-rays or digitally postprocessed images. A markedly enlarged left atrium may even project past the right cardiac border. On the left side, the enlarged left atrium may fill out the cardiac waist or create a lateral protrusion. The esophagus below the tracheal bifurcation is displaced by a markedly dilated left atrium to the right and only infrequently to the left. Extreme dilatation of the left atrium may displace the left main bronchus upward or even narrow it, causing an increase in the tracheal bifurcation angle (normally ~70°). Enlargement of the left atrium is most easily detected in radiographs following routine opacification of the esophagus (Figs. 1.9, 1.10). Fig. 1.9a, b Change in cardiac shape due to enlargement of the left atrium, which displaces the opacified esophagus posteriorly and toward the right side. a PA radiograph. b Lateral radiograph. Fig. 1.10a, b Combined mitral valve defect with predominant stenosis. PA chest radiograph (a) and left lateral radiograph with an esophagogram (b). Enlargement of the left atrium is manifested by a double contour along the right cardiac border, a prominent atrial appendage, and deep indentation and posterior curving of the opacified esophagus. Enlarged cardiac chambers may displace other normal-sized cardiac segments in ways that are difficult to interpret. For example, a significantly dilated right ventricle may displace the left ventricle posteriorly, making it difficult to assess the size of the left ventricle in the presence of a markedly enlarged right ventricle (e. g., combined mitral valve disease). Similarly, a severely dilated left atrium may displace the right ventricle forward. Even when the left atrium is definitely enlarged, this does not necessarily signify mitral valve disease. Impairment of blood flow through the mitral valve may have other causes, such as a fixed or floating atrial tumor or a large thrombus in the left atrium, resulting in obstruction of the mitral valve. In some cases this can lead to marked enlargement of the left atrium.22,49 The degree of dilatation depends on its duration and the hemodynamic effects of the lesion. Thus, echocardiography has replaced radiography for routine clinical measurements of the cardiac chambers, although chest films still provide important initial information on the nature of the underlying heart disease (Figs. 1.11, 1.12). Radiographically visible enlargement of the heart is an important indicator of heart disease. Although there is no established correlation between cardiac size and the severity of heart disease, the presence of an abnormally enlarged heart and especially its progression over time can still provide important information. Cardiac size is determined on a standard chest radiograph with a 2-m film-focus distance, which will display the heart in its approximate actual size. Cardiac size is determined by measuring the transverse diameter of the heart, i. e., the horizontal distance between the outermost right and left cardiac borders on the P-A chest radiograph measured from the midline. The ratio of the transverse cardiac diameter to the transverse thoracic diameter (measured between the inner rib margins at the level of the right hemidiaphragm at normal inspiration) is termed the cardiothoracic (CT) ratio. The CT ratio for a normal-sized heart should not exceed 1:2. When the heart is viewed in supine radiographs (e. g., taken under ICU conditions), it appears enlarged owing to the decreased film–focus distance (1 m) and greater object–film distance (posterior cassette). The elevated position of the diaphragm also causes apparent transverse broadening of the heart. Thus, in evaluating the size of the heart (e. g., after cardiac surgery) it is helpful, though not strictly necessary, to have a preoperative bedside radiograph available to enable a meticulous comparison of the pre- and postoperative chest radiographs.60 The CT ratio in supine radiographs is of limited value in determining cardiac size, but can be useful in follow-up examinations. Since the advent of sonographic, angiographic, computed tomographic, and magnetic resonance methods for determining cardiac volume and the size of the individual cardiac chambers, chest radiographs have become obsolete for cardiac volumetry. Fig. 1.11a–f Schematic representation of the change in cardiac configuration associated with acquired valvular diseases (from Bücheler E, Lackner K-J, Thelen M. Einführung in die Radiologie. Thieme, Stuttgart, 2006. Fig. 6.23, p. 350). a Mitral stenosis. b Mitral insufficiency. c Aortic valve disease. d Tricuspid stenosis. e Tricuspid insufficiency. f Pulmonary valve disease. Fig. 1.12a, b Multivalvular disease (combined aortic, mitral, and tricuspid valve disease). a PA radiograph b Lateral radiograph with esophagogram. • Prominent right cardiac border due to enlargement of the right atrium (RA). • Significantly enlarged left atrium (LA) causes convexity of the cardiac waist and is viewed as a faint double contour on the right cardiac border. • Massive cardiac dilatation is also present at the ventricular level. • Lateral radiograph shows right ventricular outflow tract dilatation and displacement toward the retrocardiac space. • The left ventricle occupies the retrocardiac space completely. • The esophagus is curved posteriorly by the enlarged left atrium. • Calcification of the aortic (AV) and mitral (MV) valves. Ventricular hypertrophy is the basic mechanism through which the heart compensates for an increased load.61–63 Pressure overload on the heart is characterized by an increase in systolic wall tension. The initial cardiac manifestation is wall thickening, followed by concentric myocardial hypertrophy with an associated reduction of inner volume. Some dilatation may also occur as an adaptive response to chronic pressure overload, although it does not yet constitute myogenic dilatation. Hypertrophy is always the dominant initial response of the heart to an isolated pressure overload. Volume overload, characterized by increased diastolic wall tension of the dilated ventricles, leads to elongation of the myocardial fibers followed by eccentric hypertrophy with an expanded inner volume. Volume overload causes less wall thickening than pressure overload. The dominant feature of volume overload is therefore dilatation, while hypertrophy is secondary.64 Tables 1.1 and 1.2 list the most common underlying diseases leading to left- and right-sided cardiac enlargement, illustrated here for diseases causing a pressure or volume overload. Because of the low intravascular pressures in the pulmonary circulation, gravity has a much greater effect on blood flow in the lung than in the systemic circulation. Hydrostatic pressures are added to the intravascular pressures in the lower lung zones. Because of these effects, blood flow in the lower lung zones is three times greater than in the upper zones. Accordingly, pulmonary vascular markings appear larger in the basal than in the apical lung in the P-A radiograph. A reduction in the caliber of the basal vessels indicates a redistribution of blood flow that may signify the presence of pulmonary or cardiac disease. This phenomenon, described as upper lobe blood diversion is, however, appreciated only in upright P-A radiographs (Table 1.3, Fig. 1.13).
Clinical Manifestations of Heart Diseases
Chest Pain
 Essential Point
Essential Point
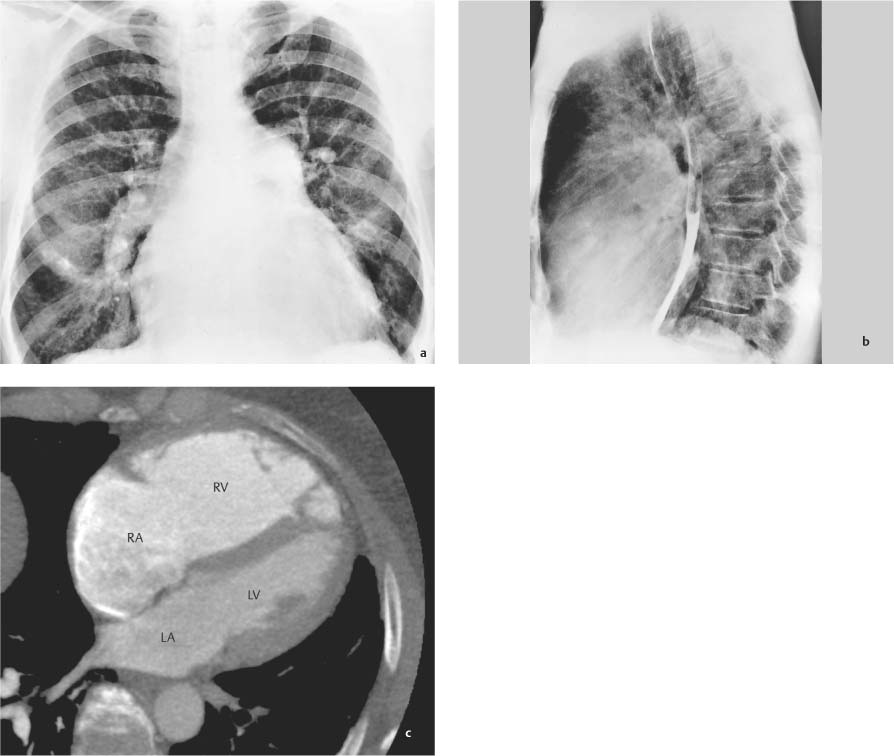
Edema
Dyspnea
Cyanosis
Arrhythmias
Hypertension
Hypotension
Syncope
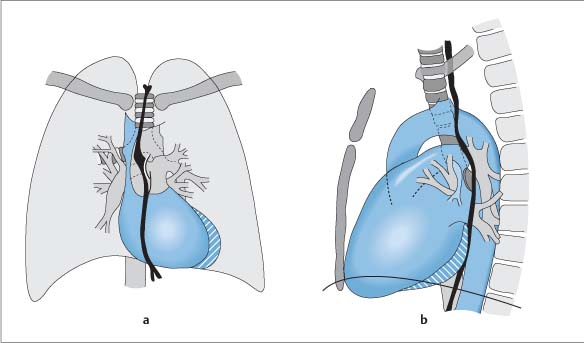
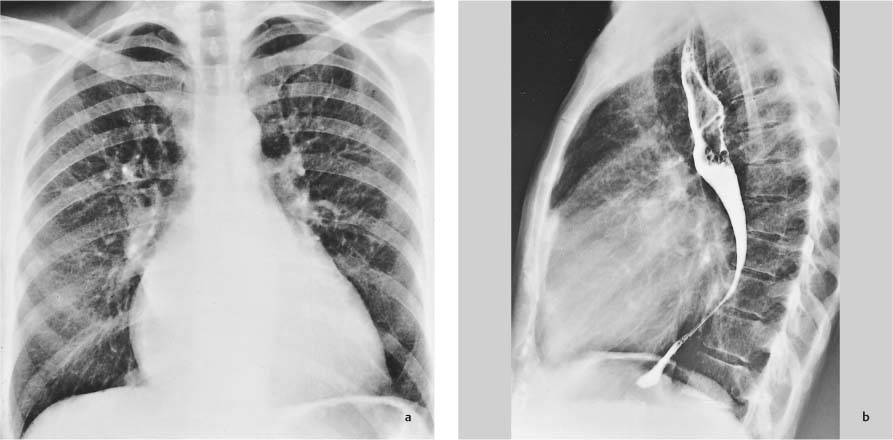
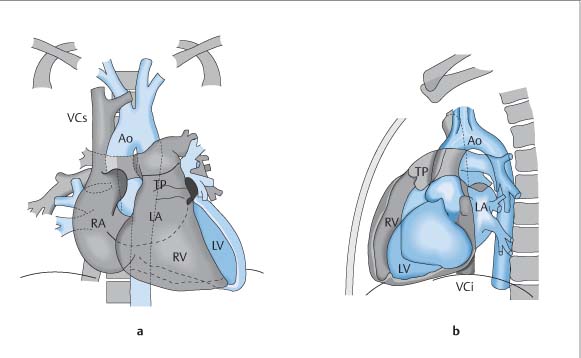
Radiographic Anatomy
Frontal (P-A) Radiograph
Lateral Radiograph
Radiographic Technique
P-A View
Oblique Views24
Contrast Radiography of the Esophagus
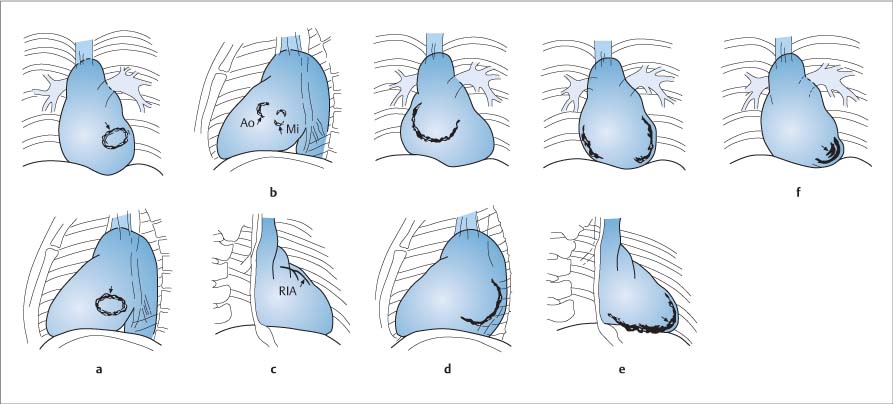
Preliminary Fluoroscopy27,28
Enlargement of the Cardiac Chambers
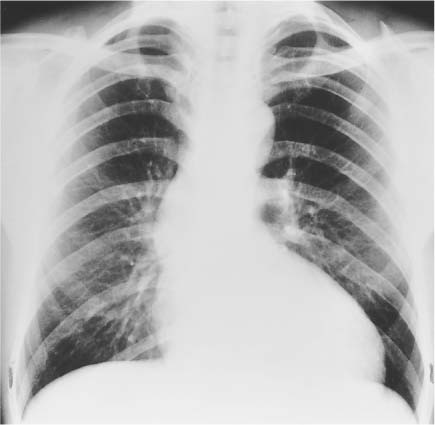
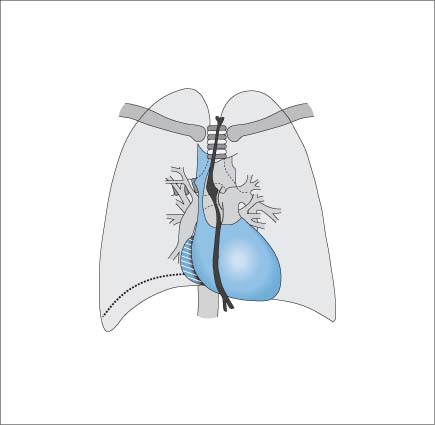
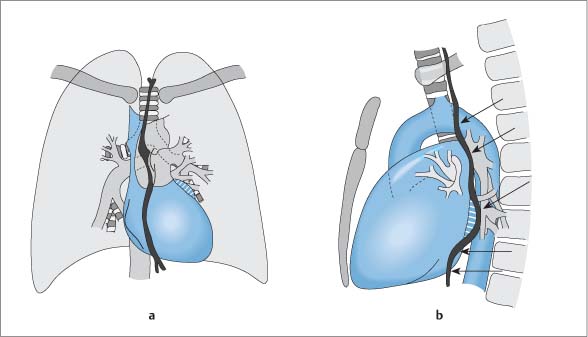
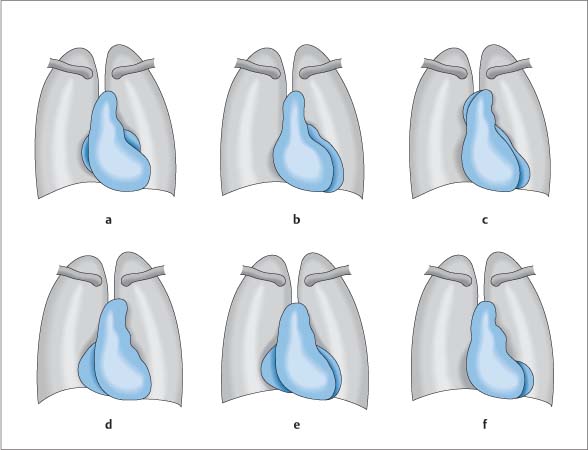
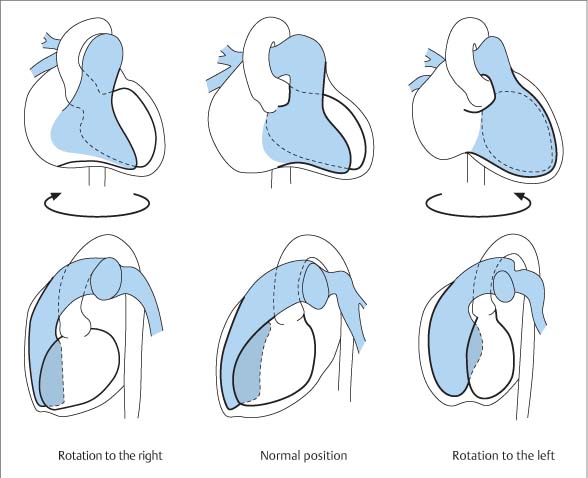
Right Ventricle
 Essential Point
Essential Point
Left Ventricle29–31
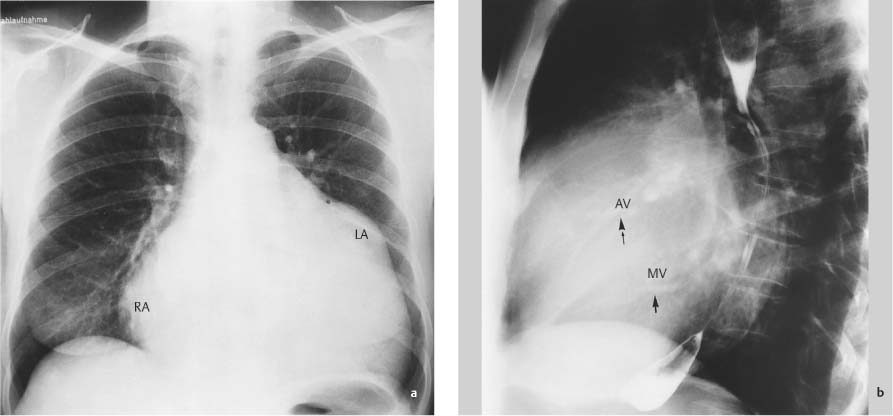
Right Atrium32
Left Atrium33–38
Combined Change39–48
Radiographic Determination of Cardiac Size50–59
 Essential Point
Essential Point
Cardiac Hypertrophy and Dilatation
Pressure Overload
Volume Overload
Underlying Diseases
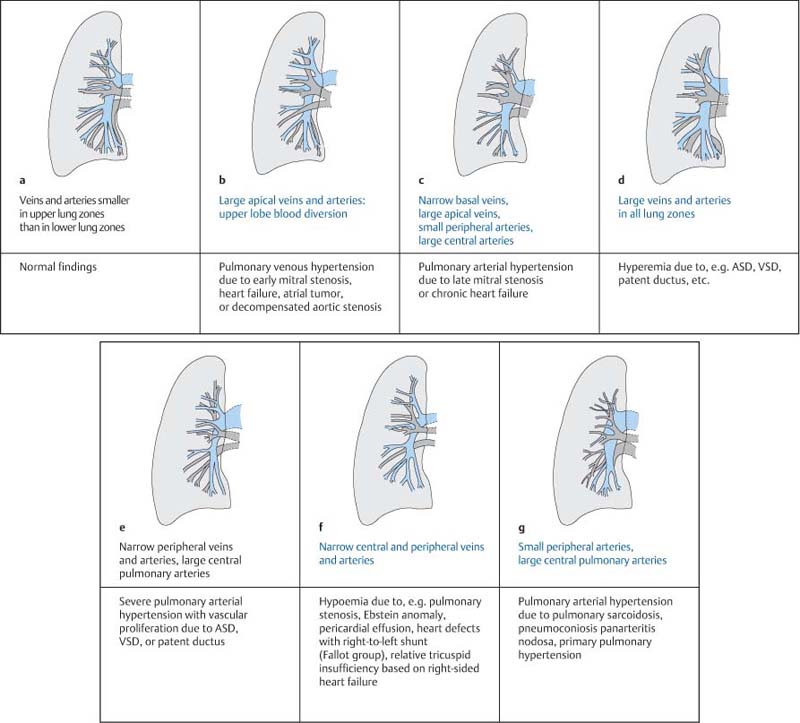
Importance of Pulmonary Vasculature in Cardiac Diagnosis18,65
Pressure overload • Aortic stenosis • Coarctation of aorta • Systemic arterial hypertension Volume overload • Aortic insufficiency • Mitral insufficiency • Ventricular septal defect • Patent ductus arteriosus • Aortopulmonary window • AV fistula |
Pressure overload of the right ventricle Frequent causes—pulmonary arterial hypertension due to: • Primary parenchymal lung disease • Primary or secondary pulmonary vascular obstruction • Shunt lesions • Severe mitral valve disease • Severe aortic valve disease • Pulmonary congestion due to left-sided heart failure Rare causes: • Pulmonary stenosis Volume overload of the right ventricle Frequent causes: • Atrial septal defect • Anomalous pulmonary venous return Rare causes: • Tricuspid insufficiency • Pulmonary insufficiency • Ventricular septal defect |
Pulmonary Congestion
When normal venous return from the lungs to the heart is impaired, this leads to congestion of blood flow in the pulmonary veins. The earliest sign of this process is a visible increase in the caliber of the upper lobe veins. The increase in left ventricular filling pressure leads to a rise of pulmonary venous and pulmonary capillary pressure, altering the balance between capillary fluid extravasation and reabsorption in favor of extravasation. The resulting increase in tissue pressure eventually leads to a reduction of blood flow in the basal lung zones. This redistribution of blood flow is manifested by enlarged arteries and veins in the upper lung zones and by small vessels in the lower zones. This consequence of decreased left ventricular inflow can be appreciated in the plain chest radiograph, which also shows enlargement of the left atrium.
In patients with severe mitral valve stenosis or chronic heart failure (Table 1.4), there is an additional active arterial vasoconstriction with intimal proliferation, leading to a rise in pulmonary arterial pressure.66–68 Radiographs show signs of pulmonary arterial hypertension with large central pulmonary arteries and narrow peripheral arteries. As this state continues to progress, marked by a growing imbalance between fluid extravasation and reabsorption, fluid begins to accumulate in the pulmonary interstitium. This is followed by the entry of fluid into the alveoli and finally into the bronchioles. As this occurs, the clinical picture progresses from acute dyspnea to manifest pulmonary edema (Table 1.5, Fig. 1.14).69
Large apical veins and arteries (upper lobe blood diversion) | Pulmonary venous hypertension due to early mitral stenosis, heart failure, or atrial tumor |
Narrow basal veins, large apical veins, small peripheral arteries, large central arteries | Pulmonary arterial hypertension due to late mitral stenosis, chronic heart failure |
Large arteries and veins in all lung zones | Hyperemia due to ASD, VSD, ductus arteriosus, etc. |
Narrow peripheral arteries and veins, large central pulmonary arteries | Severe pulmonary arterial hypertension with vascular proliferation due to ASD, VSD, or ductus arteriosus |
Small central and peripheral arteries and veins | Hypemia due, for example to pulmonary stenosis, Ebstein anomaly, pericardial effusion, heart defects with a right-to-left shunt (Fallot group), relative tricuspid insufficiency based on right-sided heart failure |
Narrow peripheral arteries, large central pulmonary arteries | Pulmonary arterial hypertension due to pulmonary sarcoidosis, pneumoconiosis, panarteritis nodosa, primary pulmonary hypertension |
• Loss of contractile muscle mass (myocardial infarction, coronary heart disease) • Persistent pressure overload (hypertension, aortic stenosis, coarctation of the aorta, pulmonary hypertension, pulmonary stenosis, congenital heart disease with elevated right ventricular pressure) • Persistent volume overload (valvular insufficiency, congenital heart disease, vascular malformations with right-to-left shunt, arteriovenous fistulas, Paget disease) • Abnormal heart rate (hypercritical tachycardia, abnormal bradycardia, arrhythmias) • Noncoronary cardiomyopathy (myocarditis, dilated cardiomyopathy, toxic and metabolic cardiomyopathies, endocrine myocardial alteration) • Impaired ventricular filling (restrictive cardiomyopathies, constrictive pericarditis, storage diseases, cardiac tumors, cardiac thrombi) |
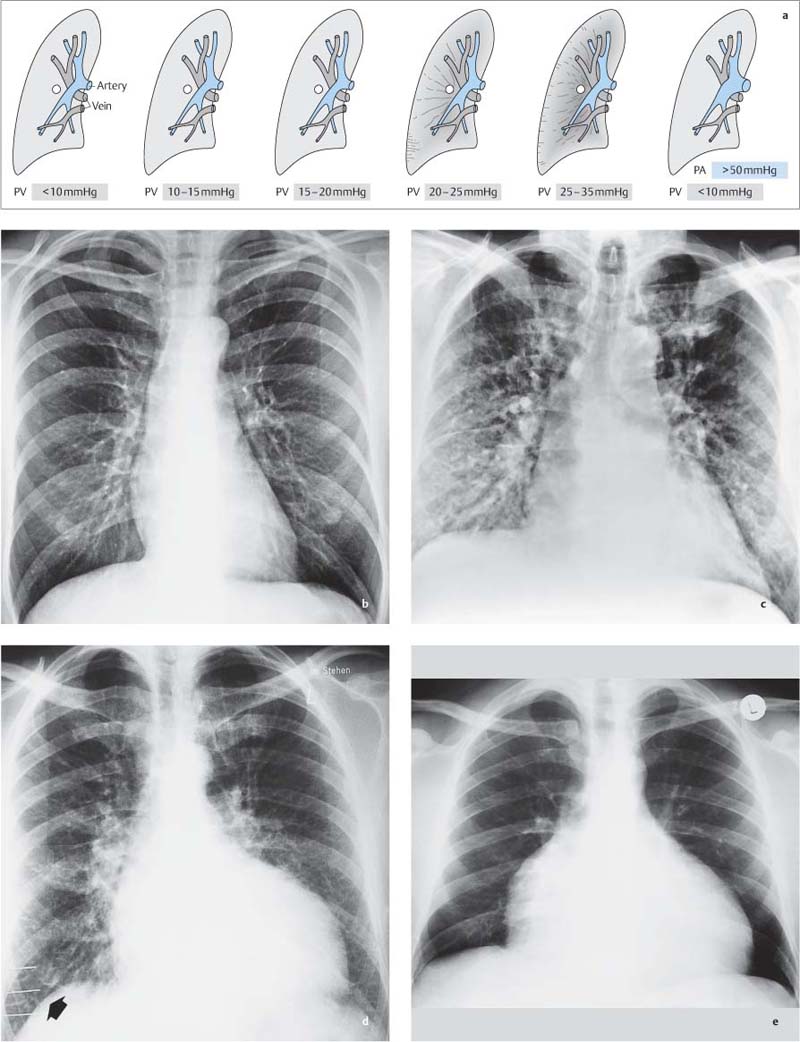
Fig. 1.13a–g Changes in pulmonary vasculature due to altered pulmonary hemodynamics (gray = vein, blue = artery).
a Normal vascular pattern.
b Pulmonary venous congestion with large upper lobe veins and reduced calibers of lower lobe vessels.
c Pulmonary arterial hypertension due to chronic pulmonary congestion, with large central pulmonary arteries, slightly enlarged upper lobe veins, and narrow peripheral arteries and veins.
d Pulmonary hyperemia with enlargement of pulmonary arteries and peripheral arteries and veins due to increased blood flow.
e Pulmonary arterial hypertension as a result of persistent hyperemia.
f Small peripheral and central vessels due to decreased pulmonary blood flow.
g Pulmonary arterial hypertension (parenchymal cor pulmonale) with small peripheral vessels and large central pulmonary arteries.
Myocardium | • Myocardial infarction • Septal rupture • Advanced cardiomyopathy • Myocarditis |
Valve apparatus | • Decompensated valvular heart disease • Papillary muscle rupture • Chordae tendineae rupture |
Pericardium | • Ventricular tamponade due to hemopericardium • Ventricular wall rupture • Effusion |
Cardiac rhythm | • Severe tachycardia • Bradycardia |
Outflow tract | • Hypertensive crisis • Massive pulmonary artery embolism • Dissecting aortic aneurysm |
Fig. 1.14a–e a Radiographic changes in the pulmonary vasculature and interstitium resulting from increased pulmonary venous pressure (diagrammatic representation).
b Normal pulmonary blood flow.
c Pulmonary hyperemia. Ventricular septal defect due to postinfarction rupture of the septum.
d Pulmonary congestion with Kerley B lines (arrow).
e Decreased pulmonary vascular markings with small, indistinct peripheral pulmonary vessels (hyperlucent lung) in a patient with hemodynamically significant pericardial effusion.
Decreased Pulmonary Blood Flow
The lung may show signs of diminished blood flow with small central and peripheral vessels in the frontal chest radiograph, indicating a decrease in the blood supply to the lung. Possible causes include hemodynamically significant pulmonary stenosis, congenital heart defects with a primary right-to-left shunt (e. g., Fallot group), Ebstein anomaly, rare primary tricuspid insufficiency, hemodynamically significant pleural effusion, and relative tricuspid insufficiency resulting from global heart failure (Table 1.6).
Pulmonary Hyperemia
A general increase in pulmonary blood flow (active pulmonary hyperemia, not to be confused with pulmonary congestion) due to intracardiac or extracardiac shunting of blood leads to volume overload, causing dilatation of all the pulmonary arteries and veins. Over time, this increased pulmonary blood flow induces endothelial hyperplasia at the arteriolar level with intimal fibrosis in the small vessels. The subsequent rise in vascular resistance eventually leads to pulmonary arterial hypertension, with large central pulmonary arteries, narrow peripheral arteries, and a reversal of the cardiac shunt (Table 1.7).
Pulmonary Arterial Hypertension (Cor Pulmonale)70,71
The cardiac causes of pulmonary arterial hypertension are to be distinguished from pulmonary hypertension resulting from a primary lung disease (Table 1.8; see Other Forms of Pulmonary Arterial Hypertension, Chapter 12, p. 256). The signs of pulmonary arterial hypertension in these cases (large central pulmonary arteries with peripheral pruning of vessels) are accompanied by signs of the underlying disease. The central pulmonary arteries are considered to be enlarged if the width of the right descending pulmonary artery is greater than 16 mm. In a chronic obstructive syndrome with emphysema, the lung shows a generalized increase in radiolucency (Table 1.9). This state is characterized by a destruction of interalveolar septa and loss of the capillary bed, leading to precapillary pulmonary arterial hypertension. The pulmonary hypertension that develops in interstitial lung disease (e. g., sarcoidosis, pneumoconiosis) or panarteritis nodosa of the lung is based on a decrease in pulmonary vasculature. The peripheral arterial branches appear narrowed, and the veins are also reduced in caliber as a result of diminished venous return. The pulmonary hypertension imposes an increased pressure load on the right ventricle, leading by definition to a state of cor pulmonale.
• Heart defects with a right-to-left shunt (Fallot symptom complex) • Hemodynamically significant pulmonary stenosis • Ebstein anomaly • Organic tricuspid insufficiency • Relative tricuspid insufficiency developing as a result of global heart failure • Hemodynamically significant pericardial effusion |
Intracardiac | • Ventricular septal defect (including septal rupture after myocardial infarction) • Atrial septal defect • Anomalous pulmonary venous return |
Extracardiac | • Patent ductus arteriosus • Aortopulmonary window Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
 Essential Point
Essential Point