Fig. 1
Origin of all different types of ovarian tumors
Most consist of ovarian cell types, but testicular (Sertoli cell) and mixed ovarian and testicular (gynandroblastoma) cell types can occur.
Epidemiology
Sex cord-stromal ovarian neoplasms are rare, and the incidence is estimated around 0.2 per 100,000 women [1].
They are a mix of benign and malignant neoplasms but comprise only 1.2 % of the ovarian cancers, most of them (1 % out of the 1.2 %) being granulosa cell tumors.
In comparison with epithelial ovarian cancer, malignant sex cord-stromal tumors are rare, are found in younger patients, are diagnosed at an earlier stage, and may produce estrogens or androgens. These sex steroid hormones not only result in particular symptoms but may also function as tumor markers and will help in the diagnosis [2, 3]. Sex cord-stromal neoplasms have no known association with the BRCA germline mutations or a genetic predisposition to breast cancer.
In this chapter we will describe the most important and relevant sex cord-stromal tumors. Most clinicopathological information was found in Blaustein’s pathology [4] and in the pictorial essays that were published by the IOTA group [5, 6]. The sonographic features are described following the IOTA (International Ovarian Tumor Analysis) terms and definitions [7].
Granulosa Cell Tumors
Epidemiology
The granulosa cell tumor (GCT) is a rare tumor that accounts for 1–3 % of all ovarian tumors, but is the most common sex cord-stromal tumor (70 %) and the most common (80 %) hormone-producing ovarian tumor [8–11].
The incidence is 0.5–1.5/100,000 women per year [12]. One-third of granulosa cell tumors occur in premenopausal women, more than 50 % in postmenopausal women and 5 % in the prepubertal period [11]. It is widely thought that granulosa cell tumors develop as a result of stimulation of the granulosa cells but the stimulus is unknown. Radiation therapy, damage to the ova and granulosa cells, increased release of gonadotropins during fertility treatment, and the use of tamoxifen (due to the estrogenic activity caused by the estrogenic metabolites) have all been proposed to be potential causal factors, but there is no real evidence to support any of these hypotheses [13]. There are two types of GCTs, the adult type and the juvenile type (Fig. 2). The juvenile type comprises only 5 % of granulosa cell tumors [11, 14]. The differences between both types are merely histopathological (Table 1).
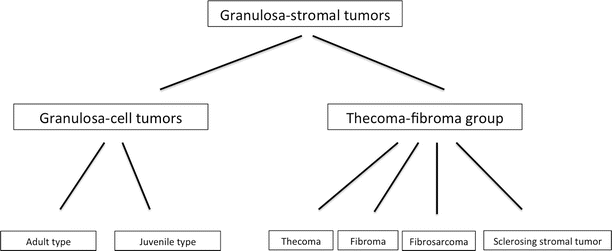

Fig. 2
Classification of the granulosa-stromal tumors
Table 1
Comparison between the adult and juvenile type granulosa cell tumor
Adult GCT | Juvenile GCT | |
|---|---|---|
Incidence | 1–3 % of all ovarian tumors | 5 % of all GCTs |
95 % of all GCTs | ||
Benign or malignant | Malignant | Malignant |
Prognosis | Low malignant potential | |
≥75 % stage I | ||
Late recurrence | ||
10 years survival: 60–90 % | ||
Survival is related to stage and size | ||
Recurrence | 25 % | 5 % |
Age | Postmenopausal > premenopausal | 97 % < 30 years |
50–55 years | ||
Typical features | Macroscopic | Macroscopic |
Large, lobulated, gray or yellow, solid, and cystic components, hemorrhagic areas | Same presentation as adult type | |
Microscopic | Microscopic | |
Pale nuclei, grooved (coffee bean), Call-Exner bodies, no luteinization, low mitotic activity | Dark nuclei, no grooves, Call-Exner bodies are very rare, often luteinization, high mitotic activity | |
Typical symptoms | Estrogenic symptoms: bleeding disorders, endometrial pathology (hyperplasia, adenoca (<5 %)) | Estrogenic symptoms: irregular cycle and bleeding disorders, isosexual pseudoprecocity in children |
Clinical Symptoms
A hyperestrogenic state due to the hormone production inside the tumor is often seen in patients with a granulosa cell tumor and results in clinical symptoms that facilitate the diagnosis [15]. Children usually present with isosexual precocious puberty or occasionally galactorrhea [11, 16]. Estrogen-related symptoms in adolescents or adults are bleeding disorders and breast enlargement. Depending on the diagnostic criteria, 24–80 % of the patients with a GCT have endometrial pathology [17]; 20–65 % of the endometrial pathology being endometrial hyperplasia and up to 10 % being endometrial cancer [18, 19].
Other clinical symptoms are abdominal distension and pain. This might be related to the fact that granulosa cell tumors tend to be large and that they may rupture spontaneously resulting in a painful hematoperitoneum or a hemorrhagic component inside the tumor.
Macroscopic Appearance
GCT’s are typically rather large, commonly encapsulated tumors with a smooth or lobulated surface. The tumor has a gray- to yellow-sectioned surface and is composed of solid and cystic areas in variable proportions. Especially in the large tumors, hemorrhagic areas are often found. Totally cystic lesions comprise a minority [17].
Microscopic Appearance
Two major subtypes are recognized, an adult and a juvenile type. The differences between them are mostly the age that they arise and the histopathological findings. The most important differences are described in Table 1.
Prognosis
The natural course of the GCT is that of a slow-growing tumor with a local spread that recurs rather seldom or very late, sometimes even as late as after 37 years [17, 18, 20, 21]. Therefore, it seems more appropriate to call a granulosa cell tumor a “tumor with a low malignant potential.” There is a recurrence rate of up to 25 % and most authors report an average time to recurrence that varies between 5 and 10 years. Recurrences are usually intraperitoneal. For stage I disease, the 5-year survival rate is more than 90 %, but for the more advanced stages, the 5-year survival rate varies between 0 and 22 %.
Despite their more aggressive histological appearance, only about 5 % of juvenile GCTs recur or metastasize. Their prognosis mainly depends on tumor stage. It is excellent for stage I tumors but poor at stage II to IV.
Sonographic Characteristics
The largest study describing the sonographic characteristics of GCT’s was performed by the IOTA group [6]. Twenty-three patients with a GCT were included in four international centers that all participated in the International Ovarian Tumor Analysis (IOTA) studies. The patients were all scanned using 2D color Doppler and grayscale imaging by experienced ultrasound examiners following the standardized examination technique, terms, and definitions recommended by the IOTA group for examination of adnexal masses [7]. At the end of the scan, the ultrasound examiner suggested a diagnosis of a benign or malignant mass on the basis of his or her subjective evaluation of the ultrasound findings, i.e., pattern recognition or subjective assessment. Eighteen (78 %) of the GCTs were of the adult type, 3 GCTs (13 %) were of the juvenile type, and in 2 (9 %) cases the tumor type was not defined by the pathologist. For patients with a primary GCT, all but one were stage I tumors, one patient was diagnosed with a stage IIb tumor, and three patients had a recurrence of a GCT.
The ultrasound characteristics of the granulosa cell tumors are presented in Table 2. All but one tumor contained solid components. Twelve were multilocular solid (52 %), 9 were purely solid (39 %), one was unilocular solid (4.3 %), and one was multilocular (4.3 %). Of the 13 multilocular/multilocular-solid masses, all 13 masses contained 5 locules or more and 10 out of 13 (77 %) contained 10 locules or more. The echogenicity of the cyst content was usually low level (7/16, 44 %) or mixed (6/16, 38 %). Papillary projections were found in only four patients (17 %). GCTs are large tumors. The median largest diameter was 102 mm (range 37–242). Only one mass was smaller than 50 mm, and more than 50 % of the masses were larger than 100 mm. Ascites was found in five cases (22 %).
Table 2
Overview of grayscale and color Doppler findings in 23 granulosa cell tumors included in the IOTA studies
Locularity | Unilocular | 0/23 (0 %) |
Unilocular solid | 1/23 (4.3 %) | |
Multilocular | 1/23 (4.3 %) | |
Multilocular solid | 12/23 (52 %) | |
Solid | 9/23 (39 %) | |
Number of loculesa | ≥5 | 13/13 (100 %) |
≥10 | 10/13 (77 %) | |
Presence of papillarities | 4/23 (17 %) | |
Largest diameter of lesion | Median: 102 mm | Range: 37–242 |
0–50 mm | 1 (4.3 %) | |
51–80 mm | 6 (26 %) | |
81–100 mm | 4 (17 %) | |
>100 mm | 12 (52 %) | |
Echogenicity cyst contentb | Anechoic | 2/16 (12.5 %) |
Ground glass | 0/16 (0 %) | |
Low level | 7/16 (44 %) | |
Hemorrhagic | 1/16 (6 %) | |
Mixed | 6/16 (38 %) | |
Color score | 1: No flow | 0/23 (0 %) |
2: Minimal flow | 2/23 (9 %) | |
3: Moderate flow | 13/23 (57 %) | |
4: Highly vascularized | 8/23 (35 %) | |
≥ 3 | 21/23 (91 %) | |
Ascites | 5/23 (22 %) |
Most granulosa cell tumors manifested moderate (color score 3) to high color (color score 4) content at color or power Doppler examination (color score 3 in 13/23, 57 %; color score 4 in 8/23, 35 %). GCT’s often contain hemorrhagic components.
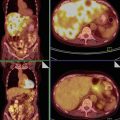
After pattern recognition, two typical patterns were described: the first pattern was a solid mass with heterogeneous echogenicity of the solid tissue as can be seen in necrotic tissue. An example of this pattern is demonstrated in Figs. 3 and 4a, b. The second pattern is a multilocular-solid mass containing a considerable amount of solid tissue around relatively small locules but with no papillary projections. It typically has a “Swiss cheese” appearance due to the large number of small locules with a variable thickness of solid tissue around the cystic areas (Figs. 5, 6, 7, and 8). But GCTs cannot always be assigned to either one of the groups as demonstrated in Fig. 9a, b.
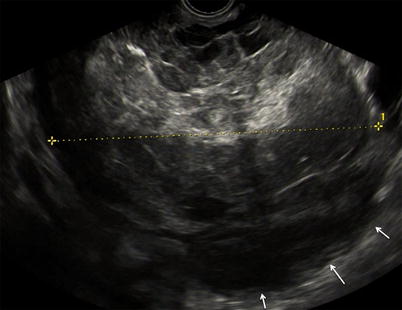
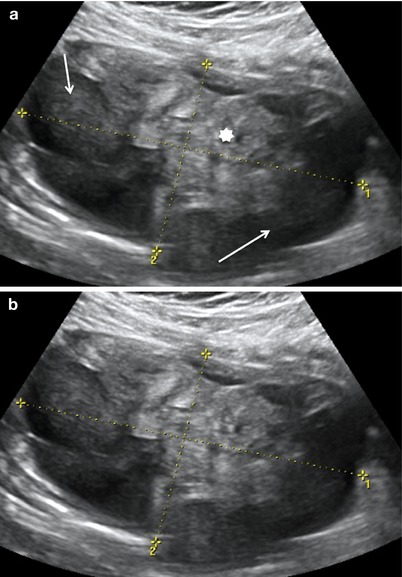
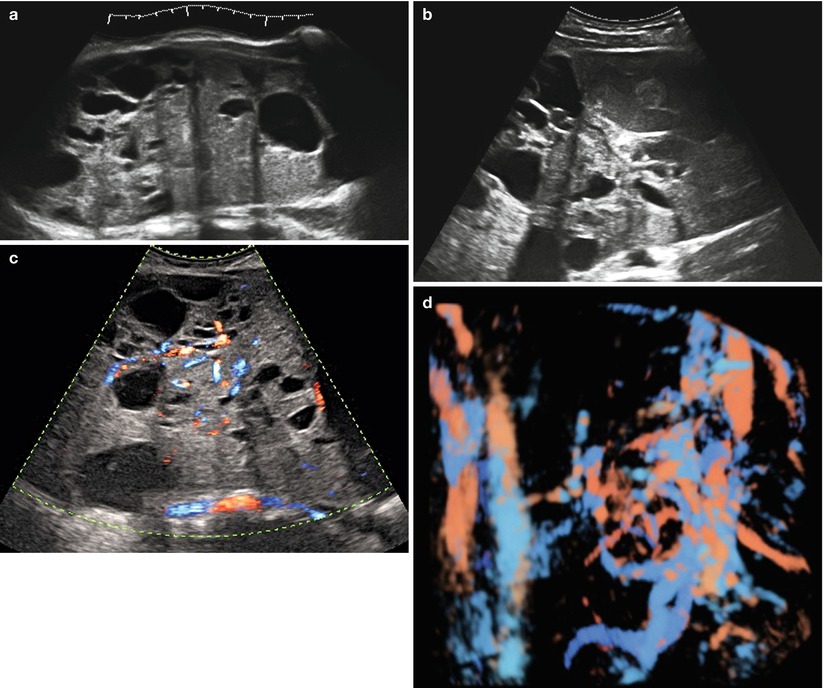

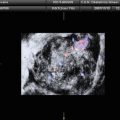
Fig. 3
Large purely solid GCT with an irregular external wall (arrows). The mixed echogenicity of the solid tissue suggests necrosis

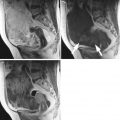
Fig. 4
(a, b) The echogenicity of this mass is in homogenous and gives the impression that large cystic components surrounding the solid tissue (★) are hemorrhagic (arrows). This GCT demonstrates the typical necrotic type

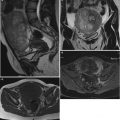
Fig. 5




(a–d) Grayscale (a, b) and color Doppler (c, d) images of a very large (174 × 127 × 206 mm) stage Ia granulosa cell tumor in a 26-year-old patient (coincidental finding during investigation for arterial hypertension). The whole of the tumor can only be measured using the extended view modus (a). Figure (d) is a rendered 3D HD flow Doppler image demonstrating the vascular tree of the tumor
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree