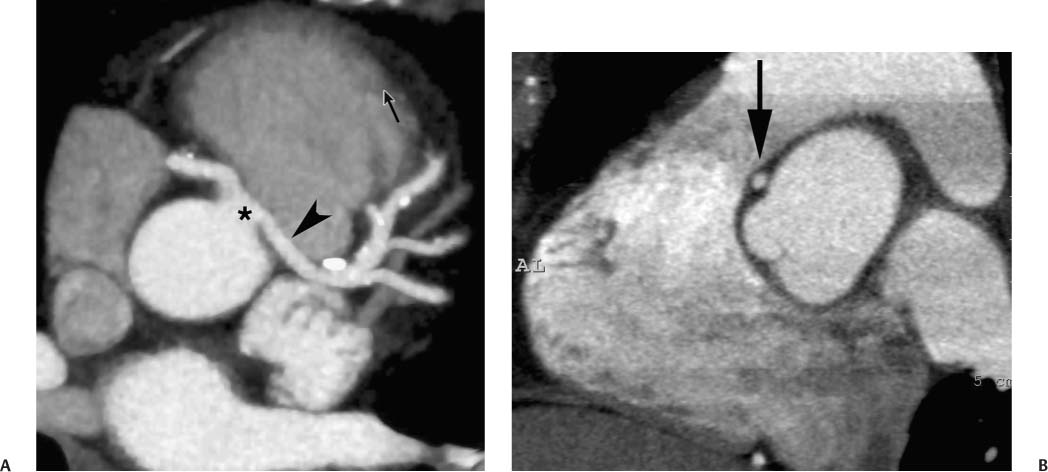
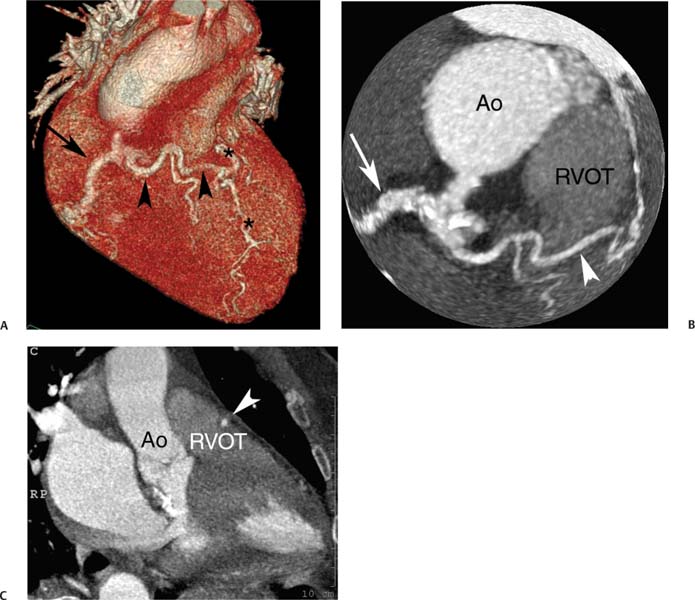
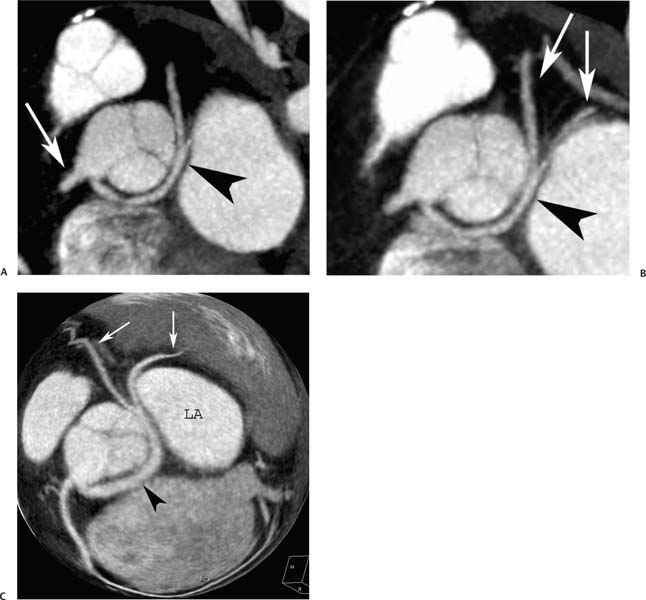
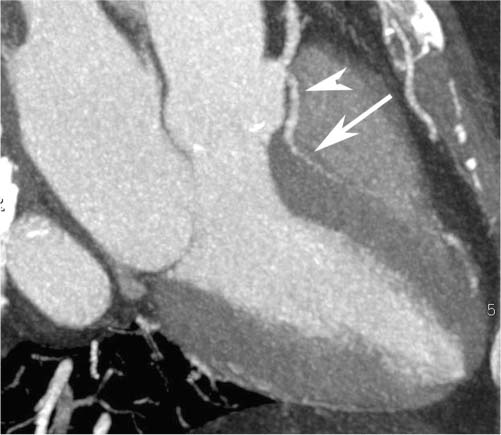
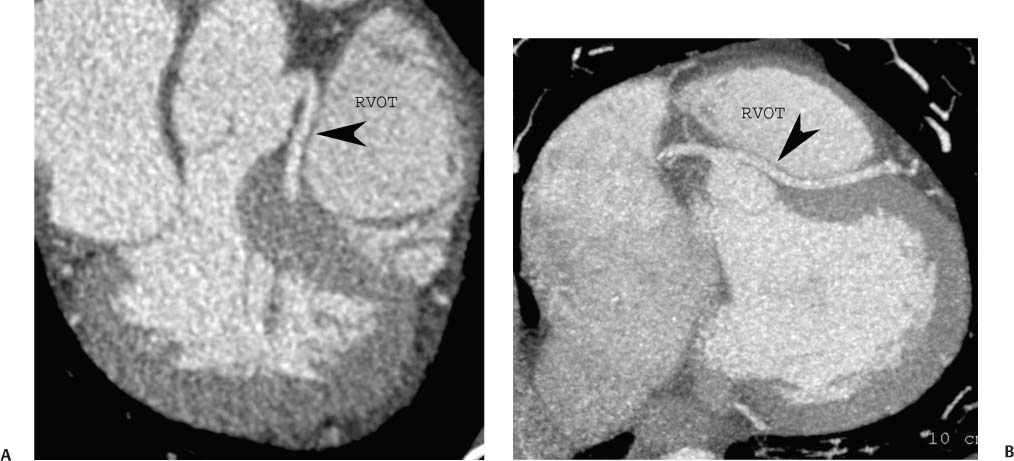
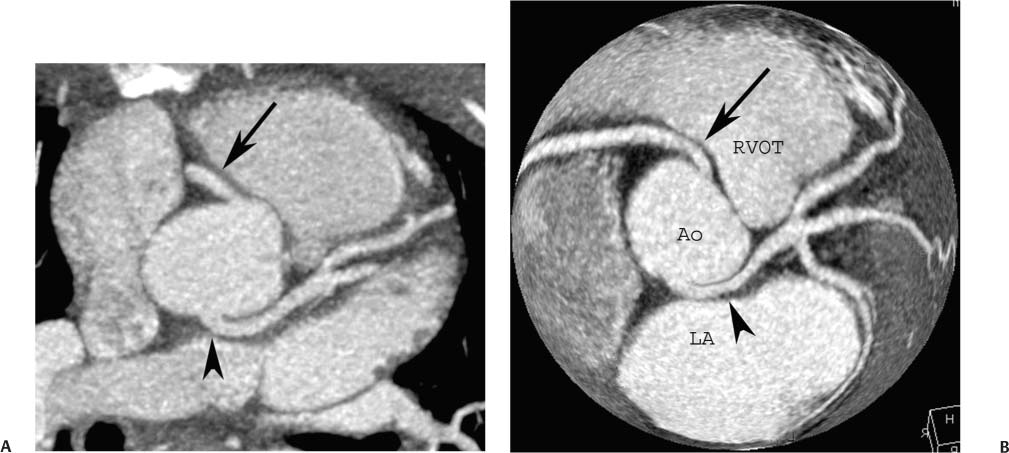
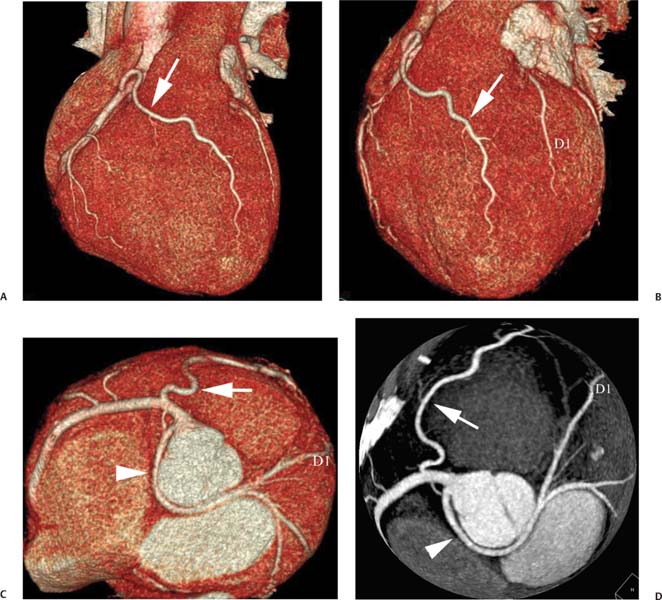
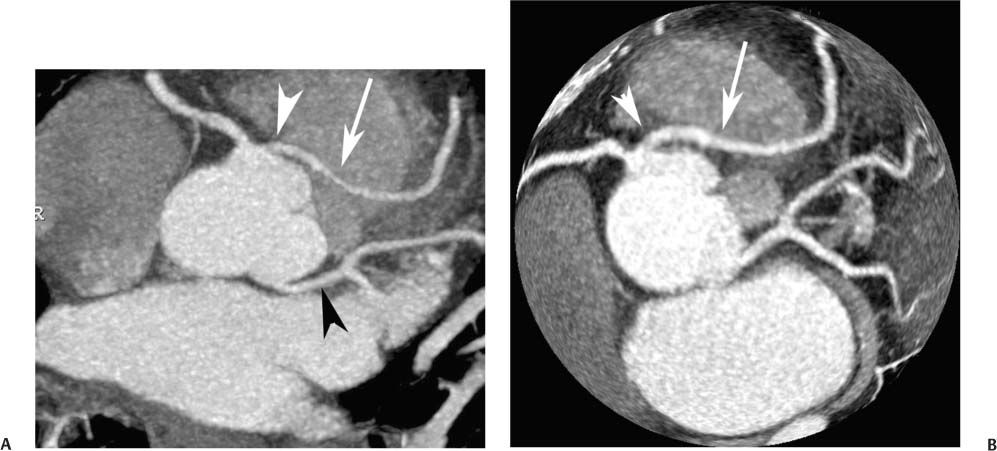
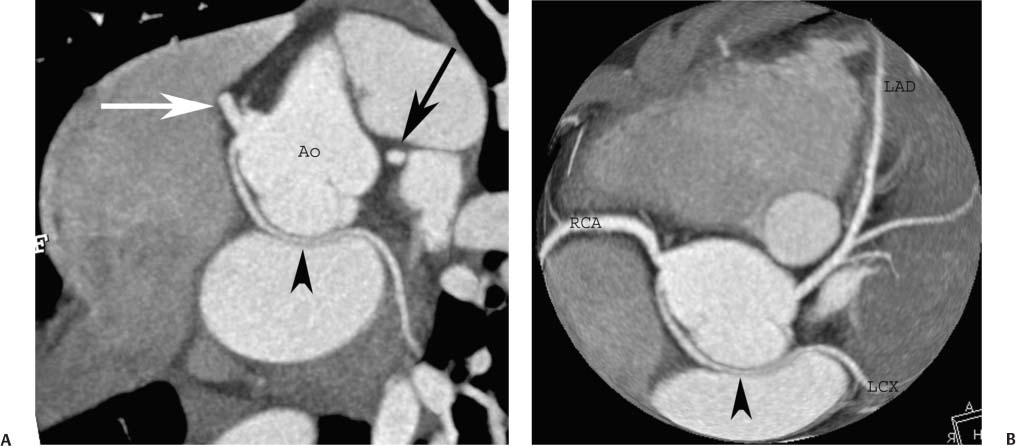
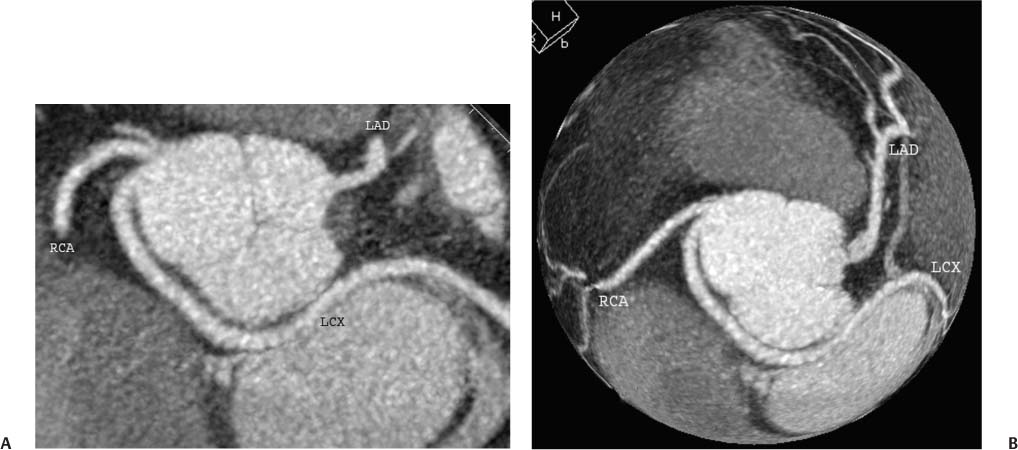
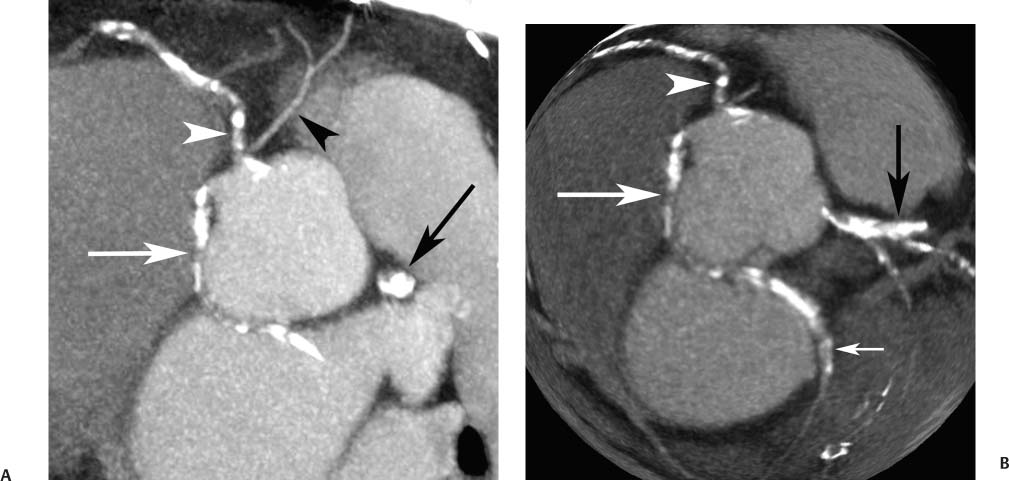
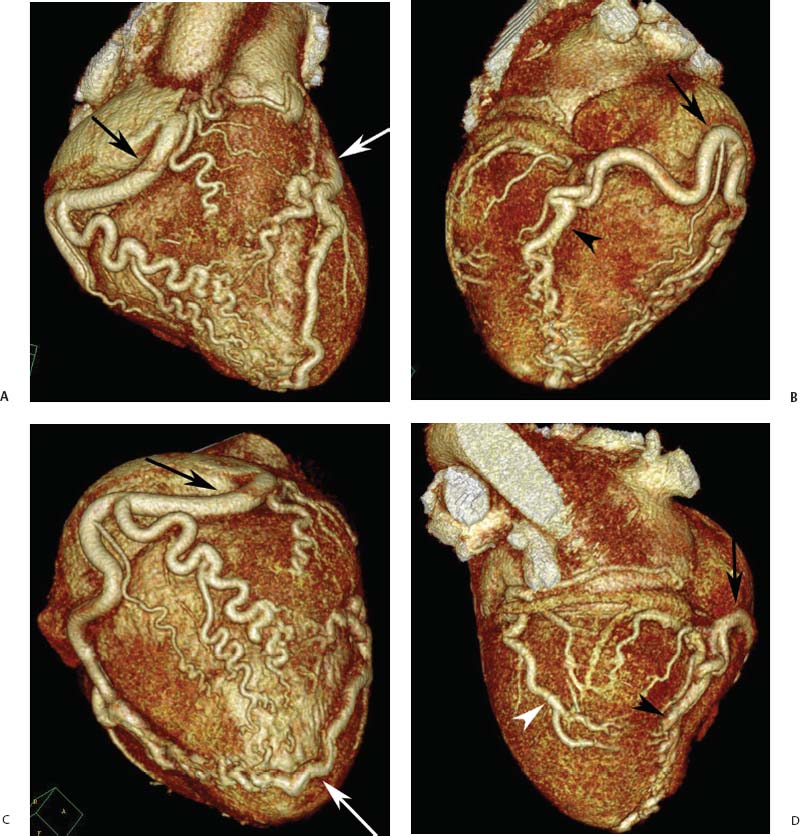
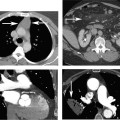
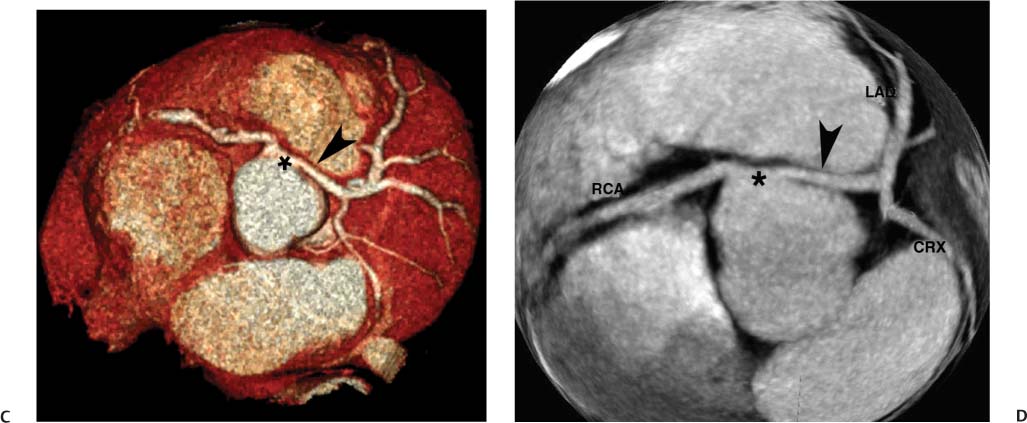
4 Coronary anomalies have been implicated as the cause of sudden death in up to 19% of athletes.1 A study of deaths in U.S. high school and college athletes reported that coronary anomalies were responsible for 11.8% of deaths.2 Among 14-to 40-year-old individuals, coronary anomalies are involved in 12% of sports-related sudden cardiac deaths.3 Hemodynamically significant coronary anomalies may manifest with angina, arrhythmia, myocardial infarction, or sudden death and may promote the onset and progression of coronary atherosclerosis.4 Unfortunately, it is often difficult to establish a definite causal relationship between coronary anomalies and clinical events. The discussion of normal coronary anatomy in the previous chapter included common coronary variations. The present chapter expands the discussion to include coronary anomalies, defined as anatomic features that are present in less than 1% of the population. Although it might be useful to classify coronary anomalies based on clinical significance, physiologic correlation is often lacking.5 Nonetheless, coronary anomalies are classified as benign or potentially serious based on anatomic considerations and anecdotal clinical observation. Coronary CT angiography (CTA) is particularly well suited to demonstrate anatomic detail for clinical evaluation and research study of coronary anomalies. Features of coronary anatomy that should be evaluated include the coronary artery ostium, course of the artery, arterial termination, and arterial size. The number of coronary ostia should be noted, and each ostium should be evaluated for location, size, and angle of origin.6 The presence of two coronary ostia, one in the right coronary sinus and one in the left coronary sinus, is a minimum requirement for normalcy.7 As discussed in the previous chapter, up to four coronary ostia may be present, including independent origins of the right coronary artery (RCA), conus artery, left anterior descending artery (LAD), and left circumflex artery (LCX). The presence of a solitary coronary ostium in the aortic root or the presence of a right and a left coronary ostia originating from a single coronary sinus is anomalous. Ectopic origin of a coronary artery above the sinotubular junction, or from a vessel other than the aorta, is anomalous. Anomalous coronary artery origins may be associated with anomalies of the aortic root (Fig. 3.1) or anomalous course of a coronary artery. In these situations, coronary flow may be compromised by narrowing of the ostium or by compression of the anomalous coronary artery between the aorta and right ventricular outflow tract. The spectrum of coronary anomalies also includes less common anatomic variations, such as distal connections to other coronary arteries (intercoronary communication) and fistulae to the cardiac chambers, to cardiac veins, or to extracardiac arteries or veins. Anomalies of coronary artery size are not defined by specific size criteria, but the diagnosis can be made in the setting of a focally atretic, hypoplastic, or ectatic coronary segment. The criteria for this diagnosis are less well defined when an entire artery is involved. In the large series reported from the Cleveland Clinic Foundation, coronary anomalies were identified in 1.3% of 126,595 patients undergoing coronary arteriography.8 Among 1686 patients with anomalies, 87% had anomalies of origin and distribution of the coronary arteries, whereas 13% had coronary artery fistulae. A frequently cited smaller study documented coronary anomalies in 5.6% of 1950 consecutive cineangiograms.9 The higher incidence in this study may be related to the author’s strict definition of coronary anomalies, to meticulous technique, or to referral bias. Some studies demonstrate a higher prevalence of coronary anomalies in men,10 whereas other studies demonstrate a higher prevalence in women.11 Most studies report the overall prevalence of coronary anomalies at about 1%, although a review of the literature suggests that the true incidence of coronary anomalies “in the general population is variable and depends on genetic and geographic factors.”12 Based on the data from the Cleveland Clinic study, clinically benign coronary anomalies are present in 1.07% of the population. The most common anomalies are absent left main coronary artery with independent origins of the LAD and LCX in 0.41% and anomalous LCX from the right coronary sinus or the RCA in 0.37%. Less common benign anomalies include anomalous origin of a coronary artery from the posterior sinus of Valsalva or from above the sinotubular junction, absent LCX (“superdominant RCA”), intercoronary communications, and small coronary artery fistulae. In the Cleveland Clinic series, potentially serious anomalies were identified in 0.26% of the population. The most common serious anomalies include origin of the RCA from the left sinus of Valsalva in 0.11%, origin of the left coronary artery (LCA) or LAD from the right sinus of Valsalva in 0.05%, and multiple or large fistulae in 0.05%. Less common serious anomalies include origin of a coronary artery from the pulmonary artery and single coronary artery. Anomalous origin of a coronary artery from the pulmonary artery involves the left main coronary in approximately 75% of cases, with the remaining cases involving the RCA or LAD. Most of these patients present in early childhood, with 75% of these patients developing symptoms in the first 4 months of life and a minority surviving into adulthood.13 The overall frequency of an anomalous coronary artery arising from a pulmonary artery was 0.01% in the Cleveland Clinic study. An anomalous origin of the LCA is most frequently from the right anterior sinus of Valsalva in a common origin with the RCA or adjacent to the ostium of the RCA. The LCA may then course anterior to the aorta in the space between the aorta and right ventricular outflow tract (Fig. 4.1), anterior to right ventricular outflow tract (Fig. 4.2), or posterior to the aorta (Fig. 4.3). When the LCA courses anterior to the aorta, a sagittal image should be obtained to distinguish an inter-arterial course from a septal course. An interarterial course is diagnosed when the anomalous vessel passes between the aorta and the right ventricular outflow tract. The inter-arterial course is considered a serious congenital anomaly14 and can present with angina, myocardial infarction, syncope, ventricular tachycardia, or sudden death.15 Various hypotheses explain these consequences based on a slit-like origin, kinking of the anomalous vessel, or compression between the right and left ventricular outflow tracts. When an anomalous LCA passes anterior to the pulmonary artery or posterior to the aorta, the variant is considered benign. Likewise, a “septal” course that passes between the great vessels below the level of the pulmonary valve is generally considered a benign variation (Fig. 4.4).16 However, coronary CTA clearly demonstrates that even when the anomalous LCA drops directly into the intraventricular septum, a short segment of the proximal LCA will usually course between the aorta and the right ventricular outflow tract at the level of the aortic root (Fig. 4.5). A recent report suggests that the distinction between the malignant interarterial variant and the benign septal variant is too simplistic.17 The course of an anomalous LCA behind the pulmonary artery is often a combination of interarterial and septal segments. An uncommon anomaly involving the main LCA is a posteriorly displaced origin of the LCA from the posterior, right-sided cusp of the aorta, which is generally designated as the noncoronary cusp (Fig. 4.6). In this benign anomaly, the left main coronary artery courses posterior to the aorta. Fig. 4.1 Anomalous left coronary artery passing between the aorta of the right ventricular pulmonary outflow tract. (A) Axial image demonstrates a common origin of the right coronary artery and left coronary artery (arrowhead) from a common trunk at the right coronary sinus of Valsalva (*). The left coronary artery passes posterior to the right ventricular outflow tract (arrowhead). (B) Sagittal reconstruction demonstrates the left coronary artery as it passes anterior to the aortic root and posterior to the pulmonic outflow tract (arrow). (C, D) Surface rendering and globe maximum intensity projection demonstrate the common origin of both coronary arteries (*). The left coronary artery (arrowhead) passes between the aortic D root and the right ventricular outflow tract. LAD, left anterior descending; RCA, right coronary artery; CRX, circumflex artery. Fig. 4.2 Anomalous left coronary artery passing anterior to the right ventricular outflow tract (RVOT). (A) Volumetric surface image demonstrates a large right coronary artery (arrow), with a left coronary artery that originates from a common ostium with the right coronary artery and courses anterior to the RVOT (arrowheads). Left anterior descending (LAD) and circumflex branches are seen to arise from the left coronary artery (asterisks). (B) Globe MIP demonstrates a single coronary artery arising from the anterior sinus of the aorta (Ao). The left coronary artery (arrowhead) originates from the right coronary artery (arrow) and courses anterior to the RVOT. (C) Slab maximum intensity projection image demonstrates that the left coronary artery (arrowhead) passes anterior to the RVOT. Fig. 4.3 Anomalous left coronary artery (LCA) passing behind the aortic root. (A) Axial image demonstrates the right coronary artery (arrow) and LCA (arrowhead), both originating from the right coronary sinus of Valsava. The LCA courses posterior to the aortic root, between the aortic root and left atrium (arrowhead). (B) Slab maximum intensity projection (MIP) demonstrates the LCA as it courses posterior to the aortic root (arrowhead) and bifurcates into the left anterior descending artery (LAD) and circumflex arteries (arrows). (C) Globe MIP image again demonstrates the LCA as it courses around the aortic root posteriorly (arrowhead). There is a long left main coronary artery, which then bifurcates into the LAD and circumflex arteries (arrows). These vessels assume their normal positions in the anterior interventricular groove and the left atrioventricular groove, respectively. LA, left atrium. Fig. 4.4 Intramyocardial “septal” course of an anomalous left coronary artery (arrowhead), which arises from the right sinus of Valsalva just below the right coronary origin but courses directly down into the myocardium of the interventricular septum (arrow). Although an anomalous course of a coronary artery anterior to the aorta is generally considered a dangerous variation, it is often stated that the intramyocardial course of the anomalous vessel is protective. Fig. 4.5 Anomalous left coronary artery with an intramyocardial course. The anomalous vessel demonstrates a short proximal segment that courses between the aortic root and right ventricular outflow tract (RVOT). (A) Slab maximum intensity projection (MIP) demonstrates an anomalous anterior origin of the left coronary artery (arrowhead) coursing directly down into the myocardium of the interventricular septum. (B) Slab MIP in a different obliquity demonstrates the proximal course of this anomalous vessel (arrowhead) between the aorta and right ventricular outflow tract. Anomalous left coronary artery with an intramyocardial course. The anomalous vessel demonstrates a short proximal segment that courses between the aortic root and right ventricular out-flow tract (RVOT). (C) Sagittal MIP near the origin of the anomalous vessel demonstrates its course between the outflow tracts (arrowhead). (D) Sagittal MIP slightly to the left of the image in (C) demonstrates the intramyocardial course of the left anterior descending artery (arrowhead). In some patients, the anomalous course may involve the LAD or LCX rather than the left main coronary. In the rare patient with no left main coronary artery, both the LAD and LCX arteries may demonstrate an anomalous course (Fig. 4.7). More commonly, an isolated anomalous LAD may be associated with a normal position of the circumflex artery (Fig. 4.8). When an anomalous LAD passes between the aorta and the right ventricular outflow tract, the imaging and clinical considerations are similar to those posed by a left main coronary artery that passes between them. An anomalous circumflex from the right sinus of Valsalva almost always passes posterior to the aorta (Fig. 4.9 and 4.10), and is not in danger of compression by the outflow tracts. However, when a patient with this anomaly undergoes cardiac surgery, the surgeon should be informed of the anomalous course to avoid accidental compression of the circumflex during valve replacement.18 Furthermore, at least one study suggests that an anomalous retroaortic circumflex artery is more likely to be affected by atherosclerotic disease (Fig. 4.11).19 Fig. 4.6 Posterior origin of the left coronary artery (LCA). (A) Axial maximum intensity projection (MIP) demonstrates the origin of the right coronary artery (RCA) (arrow) from the anterior sinus of Valsalva. The LCA (arrowhead) originates more posteriorly than normal, from the margin of the posterior or right sinus of Valsalva, which is normally the non-coronary sinus. The left main coronary artery courses between the aorta and left atrium (LA) before branching into the left anterior descending and circumflex arteries. (B) Globe MIP again demonstrates a posterior origin of the LCA (arrowhead) and also suggests that the RCA (arrow) may originate slightly more to the left than usual. The RCA courses to the right for a short distance before entering the right atrioventricular groove. RVOT, right ventricular outflow tract. Fig. 4.7 Single coronary artery originating from the anterior sinus of Valsalva. Anomalous left anterior descending artery (LAD) courses anterior to the right ventricular outflow tract (RVOT) while the anomalous circumflex passes behind the aorta (Ao). (A) Volumetric surface rendering in the anteroposterior projection demonstrates a large right coronary artery (RCA). The LAD (arrow) originates from the RCA and courses anterior to the RVOT. (B) LAO projection demonstrates the anomalous LAD with an adjacent diagonal artery (D1) that arises from an anomalous circumflex artery. (C) View from above demonstrates the LAD (arrow) as well as the anomalous circumflex (arrowhead) as they originate from the RCA. The circumflex artery courses behind the aorta and supplies a large first diagonal branch (D1). (D) Globe maximum intensity projection image demonstrates similar anatomy. Fig. 4.8 Anomalous course of the left anterior descending artery (LAD) between the aortic root and right ventricular outflow tract. (A) Slab maximum intensity projection (MIP) image just off the axial plane demonstrates the origin of the left anterior descending artery (LAD) (white arrowhead) from the right sinus of Valsalva, adjacent to the origin of the right coronary artery. The LAD appears narrowed as it courses anterior to the aorta (arrow). The circumflex artery (black arrowhead) demonstrates a normal origin from the left sinus of Valsalva. (B) Globe MIP demonstrates that the LAD is not truly narrowed as it passes behind the right ventricular outflow tract. An uncommon, serious anomaly is the anomalous origin of the LCA from the pulmonary artery, also know as ALCAPA (Fig. 4.12). Most of these patients are identified as infants or during early childhood because of myocardial ischemia or infarction or congestive heart failure, and about 90% die within the first year.20 Those patients who do survive into adulthood will have numerous collateral vessels between the left and right coronary circulations. In these cases, the RCA supplies the left coronary circulation, but it also supplies a left-to-right shunt into the pulmonary artery. Increased flow through the coronary arteries results in marked dilatation and tortuosity of numerous vessels over the surface of the myocardium. Shunt physiology may result in pulmonary hypertension. ALCAPA can present as a cause of sudden cardiac death in the adult. Fig. 4.9 Anomalous course of the circumflex artery between the aortic root and left atrium. (A) Slab maximum intensity projection (MIP) demonstrates the right coronary artery (RCA) originating from the right sinus of Valsava in a normal anterior location (white arrow). A segment of the left anterior descending artery (LAD) is identified as it enters the anterior interventricular groove (black arrow). The circumflex artery originates from the base of the RCA and courses posteriorly behind the aorta (Ao) to pass between the aortic root and left atrium (black arrowhead). (B) Globe MIP demonstrates similar anatomy but demonstrates the LAD more clearly. The circumflex artery passes between the aortic root and left atrium (arrowhead) into the left atrioventricular groove. Fig. 4.10 Anomalous course of the circumflex artery (LCX) between the aortic root and left atrium. (A) Axial image demonstrates the origins of three coronary arteries. The circumflex originates from the right sinus of Valsalva, adjacent to the right coronary artery (RCA). (B) Globe maximum intensity projection (MIP) demonstrates similar anatomy. LAD, left anterior descending artery. Fig. 4.11 Anomalous circumflex artery in a patient with severe triple vessel coronary disease. (A) The right coronary artery (RCA) is in standard location, originating from the right sinus of Valsava (white arrowhead). The conus branch originates with a common trunk from the RCA (black arrowhead). The circumflex artery originates from the right sinus of Valsava, adjacent to the RCA origin, and courses around the aortic root (white arrow) posteriorly. The proximal left coronary artery is seen adjacent to the left coronary sinus (black arrow). Diffuse vascular calcifications are identified in all three major coronary arteries. (B) Globe maximum intensity oprojection (MIP) image again demonstrates the RCA originating from the right sinus of Valsava (arrowhead). The circumflex artery originates adjacent to the RCA (arrow) and courses around the aorta posteriorly. On the globe MIP, the circumflex artery can be traced as it enters the left atrioventricular groove (small arrow).
Coronary Anomalies
 Anatomic Considerations in the Diagnosis of Coronary Anomalies
Anatomic Considerations in the Diagnosis of Coronary Anomalies
 Frequency of Coronary Anomalies
Frequency of Coronary Anomalies
 Anomalies of Origin and Course
Anomalies of Origin and Course
Left Coronary Artery
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree