Chapter 78 Most coronary artery diseases in children are congenital.1,2 A large number of anomalous arrangements of the coronary arteries exist, but only a few have clinical significance. Among acquired diseases of the coronary arteries in children, Kawasaki disease (KD) is the most common. Other diseases include sequelae from trauma, vasculitides (see Chapter 82), radiation injury after oncologic treatment, and rare cases of familial hyperlipidemia and idiopathic infantile arterial calcification. Box 78-1 outlines the two principal manifestations of coronary artery diseases, together with the diseases in which they are commonly seen in children. In clinical practice, patients presenting with syncope, arrhythmia, or near sudden death usually are referred for coronary computed tomography (CT) or magnetic resonance imaging (MRI) to diagnose a coronary anomaly, to confirm and clarify echocardiographic findings of coronary anomalies, to map out the coronary arteries for surgical planning, and to evaluate the coronary tree for aneurysms and stenosis. Catheter angiography is the gold standard for the determination of luminal coronary abnormalities, such as coronary stenosis.3 During catheterization, the arterial wall can be characterized with intravascular ultrasound,4 and the hemodynamic significance of a stenotic coronary lesion on myocardial perfusion can be measured using the fractional flow reserve technique.5 In infants, transthoracic echocardiography is the first-line imaging tool for the evaluation of structural anomalies of the proximal coronary arteries. Echocardiography is usually adequate to exclude the major types of lethal anomalous coronary arteries. Echocardiography is less useful in older children, and it cannot visualize the entire coronary vasculature. Cardiac-gated multidetector row CT angiography (CCTA) and cardiac magnetic resonance angiography (CMRA) are important noninvasive alternatives to catheter coronary angiography.6 Both techniques record three-dimensional images of the heart, with the coronary vessels shown in the context of adjacent cardiac structures. Visualization is facilitated by three-dimensional postprocessing. These capabilities have made CCTA and CMRA the preferred method for the diagnosis and characterization of anomalous coronary arteries. The greatest concern regarding CCTA is that it uses ionizing radiation (see Chapter 66). Every measure must be taken to reduce the dose of ionizing radiation delivered to a patient. First, CCTA should be undertaken only if the diagnostic goals are achievable and the results affect management of the patient’s condition. As much as possible, scanning should be limited to a single pass over the heart only. Tube voltage and tube current should be kept to a minimum for the patient’s size and acceptable image quality. If possible, the pitch factor should be adjusted to the heart rate. Prospective ECG gating and ECG dose modulation should be used when appropriate. With these measures, dose-equivalent radiation is less than 10 mSv for retrospectively gated CCTA and 3 mSv for prospective gated or nongated CCTA.7 Noise reduction with iterative reconstruction currently is being investigated as a method to achieve diagnostic images at a very low radiation dose.8 A dose equivalent to less than 1 mSv for prospectively gated or nongated CCTA likely is achievable. The lack of ionizing radiation makes CMRA an attractive imaging choice. On most clinically available magnetic resonance (MR) scanners, whole heart CMRA is built on a three-dimensional, cardiac-gated, navigated echo, T2-prepared, steady-state, free-precession sequence.9 Depending on coverage, spatial resolution, heart rate, and breathing pattern, imaging time typically is 10 to 20 minutes. For this amount of time, breath holding is impractical. Respiratory motion is managed by restricting data acquisition at a predetermined range of diaphragm position monitored in real time with the navigator echo technique. Contrast for the coronary vessels is based on the intrinsic long-T2 value of blood, and no gadolinium contrast administration is required, which is an important advantage in patients who have renal failure and the risk of gadolinium contrast–related nephrogenic systemic fibrosis.10 However, the T2-contrast mechanism is not selective, and all vascular channels are equally bright. Furthermore, other materials with a high T2 value, such as pericardial effusion and pleural effusion, are as bright as blood. This lack of differentiation sometimes can confound diagnostic interpretation. Many anatomic variations of the coronary arteries exist, and the separation of normal variants from anomalous coronary arteries is arbitrary (Fig. 78-1). In persons with conventional coronary anatomy, two main coronary arteries originate from two of the three aortic sinuses of Valsalva closest to the pulmonary trunk. The three aortic sinuses are labeled according to their attached coronary arteries: the right coronary sinus giving rise to the right coronary artery (RCA), the left coronary sinus giving rise to the left main coronary artery (LMCA), and the noncoronary sinus giving rise to no coronary branch. Figure 78-1 Coronary artery distribution diagram. After its takeoff from the right coronary sinus, the RCA courses anteriorly into the right atrioventricular groove. It gives rise to a right conal artery as the first branch 50% of the time. Otherwise, the right conal artery arises directly from the right coronary sinus. This conal artery supplies the myocardium of the right ventricular outflow tract (RVOT). In the majority of cases, a sinoatrial nodal artery branches from the proximal RCA (Fig. 78-2, A). Otherwise, it arises from the proximal left circumflex (LCx) artery. The middle portion of the RCA gives rise to one or more right ventricular branches (Fig. 78-2, B). These branches are called acute marginal branches, analogous to the obtuse marginal branches from the LCx artery. The distal portion of the RCA wraps around the inferior surface of the heart. In most people, the RCA gives rise to a posterior descending artery (PDA) (Fig. 78-2, C). The RCA then enters the “crux,” the intersection between the interventricular septum and the atrioventricular groove. At the crux, the RCA bends and forms an inverted U and then continues into the left atrioventricular groove at the bottom of the heart, where it gives off multiple posterior left ventricular arteries that supply the basilar inferior wall of the left ventricle. This pattern of the distal RCA is called a right-dominant coronary system and occurs in 85% of the population. An atrioventricular nodal branch often arises from the RCA near the apex of the inverted U (Fig. 78-2, D). The PDA gives rise to many inferior septal perforator branches that supply the inferior septum, analogous to the anterior septal perforator branches that originate from the left anterior descending (LAD) artery. Figure 78-2 Major branches of the normal right coronary artery (RCA). The LMCA originates from the left coronary sinus and courses to the left for a short distance. In most people, it bifurcates into an LAD artery and an LCx artery. In other people, the LMCA trifurcates into an LAD artery, an LCx artery, and, in between, a ramus medianus branch (Fig. 78-3, A). This branch supplies the anterior left ventricular wall. The LAD artery gives rise to two sets of vessels: an epicardial set called the diagonal branches and an intramuscular set called the anterior septal perforator branches. The diagonal branches are responsible for perfusing the anterior left ventricular wall, whereas the septal perforator branches are responsible for the anterior septum. The distal LAD artery typically wraps around the cardiac apex and terminates at the inferior wall of the apex (Fig. 78-3, B). In a minority of cases, the LCx artery gives off a sinoatrial nodal branch (Fig. 78-3, C) before it enters the left atrioventricular groove. The LCx artery then gives rise to a number of obtuse marginal branches, which supply the lateral left ventricular wall (see Fig. 78-3, B). In 10% of the population, the LCx artery supplies the posterior left ventricular branches and the PDA instead of the RCA. This system is called a left-dominant coronary system. In 5% of the population, the RCA supplies the PDA while the LCx artery supplies the posterior left ventricular branches. This system is called a co-dominant coronary system. Figure 78-3 Major branches of the normal left main coronary artery (LMCA). The true prevalence of congenital coronary artery anomalies is unknown. According to adult catheter angiographic data, 0.6% to 1.5% of patients were found to have coronary artery anomalies.11,12 The largest study of this type reviewed more than 126,000 coronary studies and revealed a prevalence of 1.3%.13 Of these anomalous cases, 80% were judged to have no clinical significance.14 Ectopic coronary origin and aberrant course is estimated to affect 1% of the population. The most common type is separate origins of the LAD and LCx arteries arising from the left sinus of Valsalva (0.41%) (Fig. 78-4, A). The next most common variation is an ectopic LCx artery arising from the right sinus or the RCA and then coursing posterior to the aorta (0.37%) (Fig. 78-4, B). Both configurations are clinically benign. Other examples of benign anomalies are an absent LCx artery with a superdominant RCA that reaches the anterior atrioventricular groove, an ectopic right or left coronary artery from the posterior sinus, and an ectopic left or right coronary origin from the ascending aorta. Clinically significant coronary artery anomalies are listed in Box 78-2. e-Figure 78-4 Common benign variants of the coronary arteries. Overview: An anomalous LMCA may originate from the right coronary sinus or from the RCA. Before bifurcating into the LAD and LCx arteries, the LMCA must travel toward the left by one of three routes: anterior to the RVOT, between the aorta and the pulmonary artery, or posterior to the aorta. Similarly, an anomalous RCA may originate from the left coronary sinus or from the LMCA. It must travel toward the right atrioventricular groove by one of these three routes. For both coronary arteries, the anterior and posterior courses are clinically benign, whereas the interarterial course—that is, between the aorta and the pulmonary artery (Fig. 78-5)—is associated with sudden death.15 An aberrant LMCA with an interarterial course is estimated to affect 0.03% to 0.05% of the population. An anomalous RCA with an interarterial course is more common, with a prevalence estimated at 0.1%. The interarterial segment of the coronary artery may be intramural within the aortic wall, intramuscular within the myocardium, or free between the aorta and the pulmonary artery. Figure 78-5 An anomalous coronary artery with an interarterial course. The association between an anomalous coronary artery with an interarterial course and sudden death in young athletes was discovered in autopsy series.16–18 Sudden death in this otherwise healthy population is rare, with an incidence estimated to be 5 in 1 million people per year,19 with anomalous coronary arteries found in up to 20% of these cases. Most autopsy series report a greater number of anomalous LMCAs than anomalous RCAs, some by a ratio of 3 : 1. Given that the anomalous RCA is three times more common, the autopsy results suggest that anomalous LMCA is a more lethal lesion. The mortality rate of these lesions is unknown, because most patients have not been diagnosed and followed up prospectively.
Coronary Artery Disease in Children
Imaging Considerations
Normal Coronary Artery Anatomy
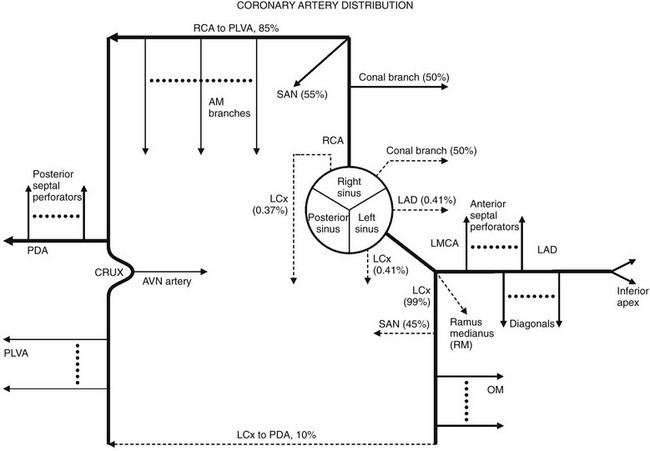
Solid lines represent the most common coronary artery pattern. Dashed lines represent common variants (prevalences in percentages). AM, Acute marginal; AVN, atrioventricular nodal; LAD, left anterior descending; LCx, left circumflex; LMCA, left main coronary artery; OM, obtuse marginal; PDA, posterior descending artery; PLVA, posterior left ventricular artery; RCA, right coronary artery; RM, ramus medianus; SAN, sinoatrial nodal.
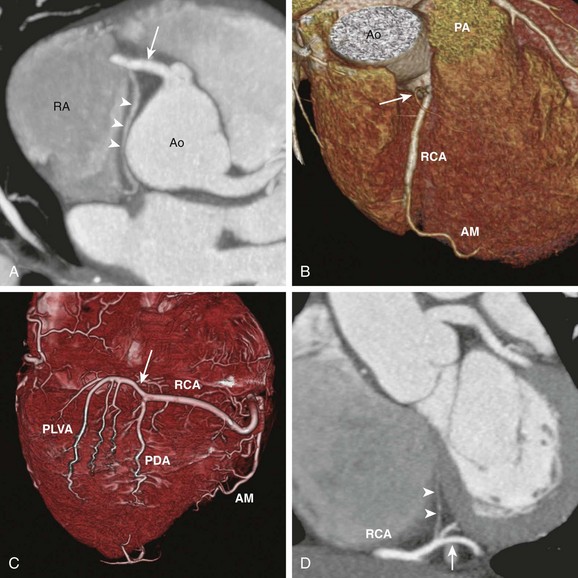
A, Axial cardiac-gated multidetector row computed tomography angiography image shows the RCA (arrow) originating from the aorta (Ao) and extending toward the right atrioventricular groove. It gives off a sinoatrial nodal branch (arrowheads) that terminates at the right atrium (RA) near the atrial septum. B, A volume-rendered image shows an acute marginal (AM) branch arising from the RCA at the right ventricular free wall. The conal branch (arrow) is the first branch of the RCA. PA, pulmonary artery. C, A volume-rendered image shows the inferior surface of a heart. The RCA gives off in succession the AM branch, the posterior descending artery (PDA), and the posterior left ventricular arteries (PLVA), which lie on the left side of the crux (arrow). D, A short-axis view shows the RCA at the crux (arrow) giving off an atrioventricular nodal branch (arrowheads).
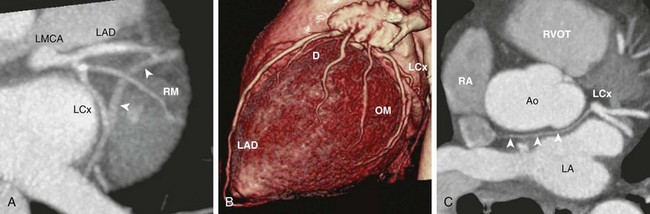
A, Oblique axial cardiac-gated multidetector row computed tomography angiography image shows the trifurcation of the LMCA into a left anterior descending (LAD) artery, a ramus medianus (RM) branch, and a left circumflex (LCx) artery. The crossing vessel (arrowheads) is the greater cardiac vein. B, A volume-rendered image shows the LAD and several diagonal (D) branches. The LAD wraps around the apex to reach the apical inferior surface. The LCx artery gives off several obtuse marginal (OM) branches. C, An axial image shows the LCx giving off a sinoatrial nodal branch (arrowheads) that travels behind the aorta (Ao) and terminates at the right atrium (RA) near the atrial septum. LA, left atrium; RVOT, right ventricular outflow tract.
Congenital Coronary Artery Anomalies
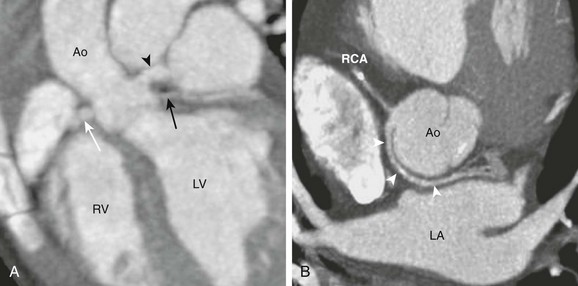
A, A horizontal long-axis view reconstructed from a coronary computed tomography angiogram of a 1-year-old boy shows separate origins for the left anterior descending (arrowhead) and the left circumflex (LCx) (black arrow) arteries. The right coronary artery (RCA) (white arrow) is in normal position. This variant occurs in 0.41% of the population. B, A short-axis view from a 40-year-old man shows the LCx artery and the RCA arising separately from the right sinus. The LCx artery (white arrowheads) wraps around posterior to the aorta (Ao) and then enters the left atrioventricular groove. This variant occurs in 0.37% of the population. LA, Left atrium; LV, left ventricle; RV, right ventricle.
Coronary Artery with Interarterial Course
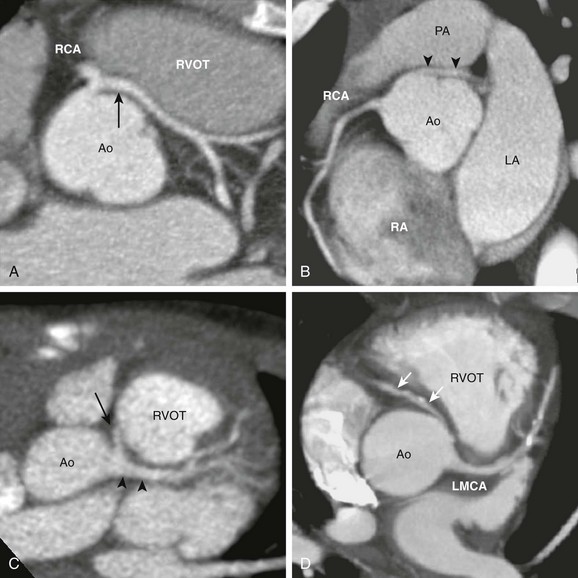
A, The axial view from a cardiac computed tomography angiogram of a 10-year-old boy shows a common origin of the right coronary artery (RCA) and the left main coronary artery (LMCA) (arrow) arising from the right sinus of the aorta (Ao). The LMCA has a long interarterial course as it travels between the aorta and the right ventricular outflow tract (RVOT). B, In this 20-year-old man, an LMCA (arrowheads) originates from the right sinus separate from the RCA, then travels between the Ao and the pulmonary artery (PA). C, In this 2-year-old boy, the RCA (arrow) arises from the LMCA (arrowheads). D, In this adult with calcific coronary plaques, an RCA (arrows) originates from the left sinus separate from the LMCA. A tapered narrowing is evident at the proximal, interarterial portion of the RCA. LA, Left atrium; RA, right atrium.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree