Detection of the joint effusion (quantity, aspect)
Detection of the synovitis (with clinically manifestation or asymptomatic)
Differentiation between active or inactive synovitis
Hyaline cartilage and fibrocartilage study (deposits)
Bone contour (erosions, osteophytes)
Tendons evaluations (deposits, tears, inflammation)
Crystal depositions evaluation (articular and/or juxta-articular spaces)
Execution of US-guided procedures (for diagnosis and/or therapeutic purposes)
Monitoring the disease evolution and progression
Differential diagnosis with other inflammatory arthritis
Gout
Gout , one of the oldest recognized diseases, is a complex form of inflammatory arthritis , first identified by the Egyptians in 2640 BC and later recognized by Hippocrates in the fifth century BC . Six centuries later, Galen was the first to describe tophi, whereas Antonie van Leeuwenhoek was the first to describe the appearance of the crystals from a gouty tophus in 1679, although their chemical composition was unknown at that time [9, 10].
Gout is the consequence of deposition of MSU crystals in joints and other tissues, affecting up to 1–2 % of men and occurs as a result of persistent hyperuricemia [11]. This is associated with excess morbidity, disability, and poorer quality of life [9]. More than 80 % of gout patients have a positive family history of gout or hyperuricemia. Four phases are described in the disease evolution: asymptomatic hyperuricemia, acute, intercritical, and chronic gout [12].
Diagnosis of suspected gout is based on typical clinical and laboratory (i.e., hyperuricemia) findings, while definitive diagnosis requires identification of MSU crystals in aspirated synovial fluid or tophi [11] .
Review of the literature revealed that the management of gout is often suboptimal. At the early stage of gout, radiography may not be useful for the disease diagnosis, as abnormal findings may take several years to develop a radiologic pattern (apart from nonspecific soft tissue swelling overlying the inflamed joint) [13] .
Nonspecific Features in Gout
Synovial Fluid
A joint effusion is an early but nonspecific finding in gout patients [12, 13, 15, 16] . During acute gout, synovial fluid may vary from complete anechogenicity (usually in an early phase of the disease) to aggregates of variable echogenicity (more often after multiple acute attacks) [15, 17–19].
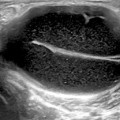
Multiple aggregates of MSU microcrystals are visualized as hyperechoic spots or bright stippled foci and gentle pressure on the skin surface makes them float in the joint cavity, creating a “snowstorm” appearance in some cases [12, 13, 15, 18–22] (Fig. 7.1).
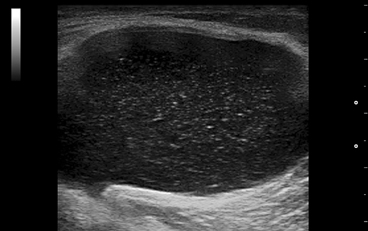

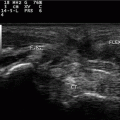
Fig. 7.1
Gout. The “snowstorm” aspect of the synovial fluid in a popliteal cyst
Gout has a predilection for the first metatarsophalangeal (MTP I) joint, with as many as 50–70 % of first gout attacks occurring there [16, 18]. Ultrasound detects more joint effusion than clinical examination especially on dorsal view of the MTP joints [23, 24].
In a recent study, the MTP I as well as the radiocarpal joints were the most frequently involved joints for hyperechoic aggregates in gouty arthritis patients (57.1 % and 38.5 % of patients, respectively). The midcarpal and the knee joints were also frequently involved in patients with gout (28.6 % and 25.3 %, respectively) [16] .
Synovial Proliferation and Hypervascularization
Similar to joint effusion, synovial hypertrophy is not specific to gout, but their association with hyperechoic spots (bright dotted foci) in the synovium is strongly evocative of gout [13, 23] .
Doppler mode (power Doppler, PD; color Doppler, CD) is able to differentiate active, inflamed, from inactive, noninflamed/fibrotic synovial tissue, by estimating the vascular pattern (Fig. 7.2).
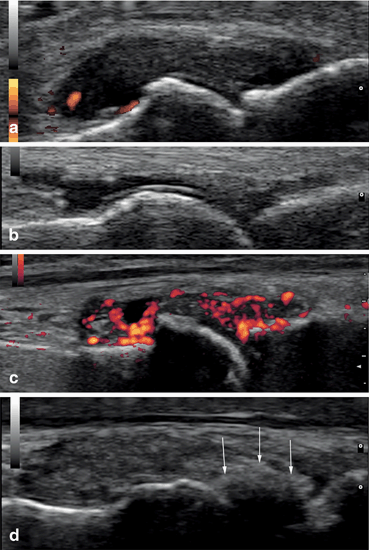

Fig. 7.2
Dorsal longitudinal scans of the first metatarsophalangeal joint in patients with gout: a acute gout—large hypoechoic content of the joint cavity, with discrete vascularization; b intercritical gout—small quantity of transonic fluid, double contour of the hyaline cartilage; c acute flare of chronic gout; d tophaceous gout—a large hard tophus (arrows) situated intra-articular. The posterior acoustic shadow makes the interosseous space non-examinable
While US features of synovial effusion, synovial proliferation, or synovial Doppler signal are not specific for gout, as these US findings are frequently found in any type of inflammatory arthritis, all of them are crucial for the diagnosis of gout and in the monitoring of the patient and therapy. In addition, PD could be a way to monitor acute gouty attacks [25, 26] .
Recent reports have shown that the PD signal disappears with treatment, which suggests that PD could be a useful method to monitor gouty synovitis response to therapy.
Interestingly, other studies reported that the PD signal was even present in asymptomatic joints from gout patients and in asymptomatic hyperuricemia [15, 25, 26], suggesting that subclinical chronic inflammation may be at work [25]. With colchicine therapy, PD signal has decreased and finally disappeared [15] .
Bone Erosions
Bone erosions on US have been defined as breaks in the hyperechoic outline of the bony cortex (step-down lesions), seen in two perpendicular planes, following the Outcome Measure in Rheumatology (OMERACT) criteria [27] .
The OMERACT MSUS group has produced a definition of erosion specific for gout as follows: intra- and/or extra-articular discontinuity of the bone surface (visible in two perpendicular planes) [28].
In gout, bone erosions are due to tophus [12–14, 18, 20, 21, 25, 29]. The tophus eroding the underlying bone is pivotal for the development of bone erosions in gouty arthritis [29], therefore the specificity of an US diagnosis of erosions is increased when there are adjacent tophi [12, 29]. Erosions have been found more commonly in patients with frequent acute gouty attacks, long disease duration, and tophi proven by US [23, 30] .
The MTP I joint (medial surface), and metacarpophalangeal (MCP) joints are the main targets for erosions in gout [14, 23, 30, 31], but they were also found commonly in MTP joints that have never been clinically affected by gout [23, 26]. Unlike erosions in rheumatoid arthritis, gouty erosions are usually deeper and more destructive [18, 30] .
US has proven to be three times more sensitive than plain films in the detection of bone erosions < 2 mm (P < 0.001) [26, 30]. So far, there is no standardized US scoring system for erosions in gout. In contrast, in rheumatoid arthritis erosions have been classified according to the bones involved (metatarsal or phalangeal), the position of the erosions (dorsal or medial) and whether they were unifocal or multifocal. They have been categorized into a semiquantitative scale as small erosion < 2 mm, moderate erosion 2–4 mm, and large erosion > 4 mm) [21, 30] (Fig. 7.3) .
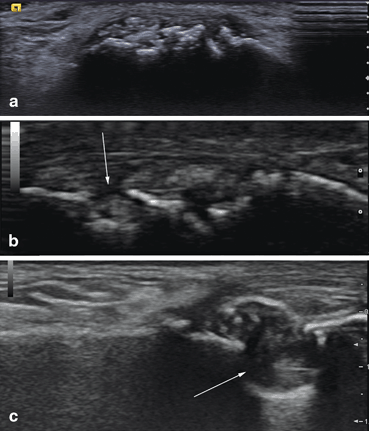

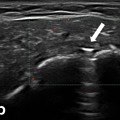
Fig. 7.3
Chronic gout. Different aspects of the erosions at the first metatarsal head: a small erosions covered with a hyperechoic and inhomogeneous material (medial scan); b large erosion (arrow) filled with hyperechoic material (tophus; dorsal scan); c very large and deep erosion (arrow) realized by an intraosseous tophus, quite similar with the radiological aspect of erosions with overhanging edges (dorsal scan). Note the posterior “dirty shadow” artifact
Specific US Features in Gout
Articular Cartilage—“Double Contour Sign”
The double contour sign (DCS) is a highly specific US feature for gout . This DCS is a result of uric acid (UA) predilection to crystallize forming deposits of MSU microcrystals on the hyaline cartilage surface, which increases the cartilage surface interface giving it a thickness similar to that of subchondral bone [18, 20, 26]. OMERACT MSUS group has given the following definition of the DCS: abnormal hyperechoic band over the superficial margin of the articular hyaline cartilage, independent of the angle of insonation and which may be either irregular or regular, continuous or intermittent and can be distinguished from the cartilage interface sign [28].
Thus, DCS is seen as an echogenic band with an irregular surface detected paralleling the bony contour with an anechoic region between. This anechoic region represents hyaline cartilage [12–14, 16, 23, 32] (Fig. 7.4) .
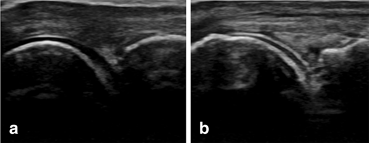

Fig. 7.4
Dorsal longitudinal scan of the metacarpophalangeal joint: a normal aspect of the hyaline cartilage; b gout— the double contour sign. Slight irregularities of the superficial margin of the hyaline cartilage can be observed
DCS adheres to the cartilage, moving together with bone and cartilage layer in dynamic moves [13–15].
There is a correlation between the presence of DCS, serum UA level, and the occurrence of UA arthropathy, which suggests that DCS could be a marker of severity [13].
DCS is most frequently detected in the dorsal aspect of the first MT head (61.5 %) and the femoral articular cartilage (41.8 %) and is seen in the majority of joints with intra-articular tophi [16, 23] .
DCS should be searched in the trochleal cartilage of knees (suprapatellar plane in maximal flexion [12, 13, 16, 23, 31] (Fig. 7.5).
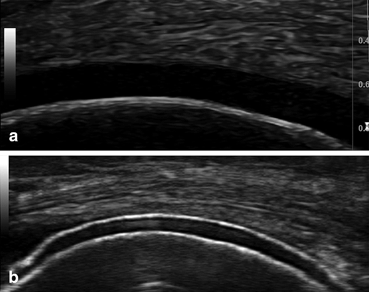

Fig. 7.5
Longitudinal scan of the femoral condyle: a normal aspect; b gout—the double contour sign
It has been reported that the frequency of the “double contour” sign in the knee is higher in clinically affected versus unaffected knees of gout patients, although the difference does not achieve statistical significance [21] . DCS has been noted in patients with an acute gout flare-up, with a history of prior gout attacks, in clinically unaffected joints, and also in patients with asymptomatic hyperuricemia. The sensitivity of this finding ranges from 25 to 95 % in patients with gout [12–18, 20, 21, 23, 25, 26, 30, 33]. False-positive results for DCS are possible in the following circumstances [13, 15, 19, 20, 23, 30, 31] :
1.
The hyperechoic aspect of the normal synovium is a regular hyperechoic band, as if written with a pen. DCS is irregular and adheres to the cartilage in dynamic moves.
2.
The joint effusion induces a posterior-wall echo reinforcement (improvement of propagation of ultrasound) and can accentuate the normal hyperechoic aspect of the synovium.
3.
A thin cartilage in small joints.
4.
Damaged cartilage associated to osteoarthritis (OA; the presence of synovial effusion in the MTP I joint, which is a quite common finding even in OA, can enhance the visualization of the superficial margin of the metatarsal hyaline cartilage).
5.
Hyper-echoic spots located in the middle layer of the cartilage and the presence of meniscal calcification are associated with calcium pyrophosphate deposition (CPPD) disease. MSU deposition in gout occurs on the surface of the hyaline cartilage producing the DCS, whereas CPPD deposition appears as a central hyperechoic focus or line within the cartilage.
Responsiveness of US to cartilage changes in gout has been demonstrated by the disappearance of the DCS in response to urate-lowering therapy (ULT) in gouty patients whose serum UA levels were lowered to 6 mg/dl for 7 months or more, but not in patients whose levels remained above 6 mg/dl [34].
A wide range of sensitivity (e.g., 21–92 %) with very high specificity (98–100 %) for US-detected DCS in gouty arthritis patients have been reported in the literature [12–14, 16, 23, 30–32]. Statistically, The was found to be present in subjects with gout significantly more often than in healthy subjects [14, 20, 23]. Inter-reader reliability assessing DCS was found to be excellent [13, 14, 21, 23, 31] .
MSU Deposits (Tophi and Aggregates)
Tophi have unique diagnostic importance to gout . They represent deposits composed of MSU crystals core, encased by dense connective tissue [35]. Earlier studies comparing US to MRI in the detection of gouty tophi revealed that MRI confirmed 81 % of the tophi reported by US and US detected 90 % of the reported tophi on MRI [7, 36].
The OMERACT MSUS group defined tophus and aggregations as follows [28]:
Tophus is a circumscribed, inhomogeneous, hyperechoic, and/or hypoechoic aggregation (that may or may not generate posterior acoustic shadow) which may be surrounded by a small anechoic rim. Aggregates are heterogeneous hyperechoic foci that maintain their high degree of reflectivity even when the gain setting is minimized or the insonation angle is changed and which occasionally may generate posterior acoustic shadow. Most commonly, tophi are hyperechoic and always heterogeneous [12–14, 16, 19, 23].
Tophi have been also described by US as “wet sugar clumps” with an oval or irregular shape [14].
Intra-articular and intrabursal tophi have been defined as heterogeneous hyperechoic (relative to subdermal fat) aggregates with poorly defined margins with or without areas with acoustic shadowing within the synovial recesses or bursae, respectively [16] .
Tophi are walled off and their contour is poorly defined [14, 37]. They could be surrounded by a thin anechoic rim. The histopathological equivalent of the anechoic rim is formed by macrophages, lymphocytes, and large foreign body giant cells [37]. This rim allows distinction of tophaceous material from proliferative synovial tissue and hyperechoic joint capsule. At the same time, this halo would be in close contact with subchondral bone in the case of pannus invasion of an erosion [14].
According to the degree of compaction of the deposits, tophi could be divided into soft/inhomogeneous echogenicity and soft to palpation/ hard/ hyperechoic aggregate generating a posterior acoustic shadow and hard to palpation/and mixed/ features of both soft and hard tophus. Tophi that are sonolucent have been termed as “soft tophi.” Hard tophi occur in longstanding tophaceous gout, and generation of posterior acoustic shadow is due to calcification within the tophus/calcified tophi [15, 17, 18, 20] (Fig. 7.6) .
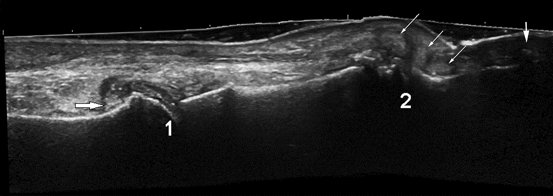

Fig. 7.6
SieScape imagine of the first toe in patient with chronic gout. Metatarsophalangeal (1) and interphalangeal (2) joints are affected. Note the soft tophi in and around the interphalangeal joint (small arrows), the hard tophus in the nail bed (large arrow), and a hyperechoic cloudy areas (horizontal arrow). Irregularities of the bone contour of the phalangeal head represent erosions
The Doppler US can make the difference between active/”hot” tophi and inactive/”cold” tophi by the presence or absence of the Doppler signal.
Nevertheless, there is a frequent presence of persistent low-grade inflammation in asymptomatic chronic tophaceous gouty arthritis on US [29]. Main sonographic features of tophi are presented in Table 7.2 .
Table 7.2
US features of Tophi
Echogenicity | Hyperechoic, hypoechoic, anechoic, and heterogeneous |
Mostly hyperechoic and heterogeneous | |
Contour | Poorly defined, could be surrounded by a small anechoic rim |
Shape | Oval (spherical or ellipsoid) or irregular |
Types according to the degree of compaction | Soft |
Hard | |
Mixed | |
Types according to the degree of inflammation | Hot |
Cold | |
Dynamic assessment | Tophi do not move with bone and cartilage |
US is able to detect tophi both inside and around the erosions [19]. Naredo et al. found that the assessment of one joint (i.e., radiocarpal joint), two tendons (i.e., patellar tendon and triceps tendon), and three articular cartilages (i.e., first metatarsal dorsal and plantar cartilage, talar cartilage, and either second metacarpal cartilage (dorsal aspect) or femoral condyle cartilage) showed the best balance between sensitivity and specificity (84.6 and 83.3 %, respectively), positive predictive value and negative predictive value (91.7 and 71.4 %, respectively) [16] .
Intra-articular Tophi
Preferred locations for the disposal of tophi are symptomatic joints and their periarticular tissue. Tophi may occur at any site but their frequent locations are toes, especially MTP I, wrist, fingers, knee, and ankle [16].
In small joints, intra-articular tophi are often associated with bone erosions.
Nearly half of the patients with gout have US-proved tophi in at least one of the examined joints, predominantly in subjects with a higher level of UA and subjects who do not receive ULT [13, 21, 23] .
Main US features of tophi in the MTP I are presented in Table 7.3 [14, 25].
Table 7.3
The main US features of tophi in the MTP I
Place of the tophi or aggregates | In the dorsal proximal recess of the synovial membrane |
Medial to the metatarsal head | |
In the central area of the joint space | |
Echogenicity | In inflamed joints, intra-articular tophi are more echogenic on the background of the hypoechoic or anechoic fluid |
Tophi and erosions | Formed, oval-shaped tophi riding on the hyaline cartilage of the metatarsal head, impinging on the proximal phalanx, and eroding into the bone |
Tophi in Periarticular Tissue
Bursae
The prepatellar and olecranon bursae are the most commonly involved sites [37].
The bursitis has chronic features of inflammation from the beginning. The walls are thick, poorly delimited from the surrounding tissues, and the cavity is always filled with proliferated synovia. There is not a good correlation with the adjacent joint involvement. Peritendinous bursae (infrapatellar, preachillian) are inflamed only in cases of concomitant tendon pathology [17] (Fig. 7.7).
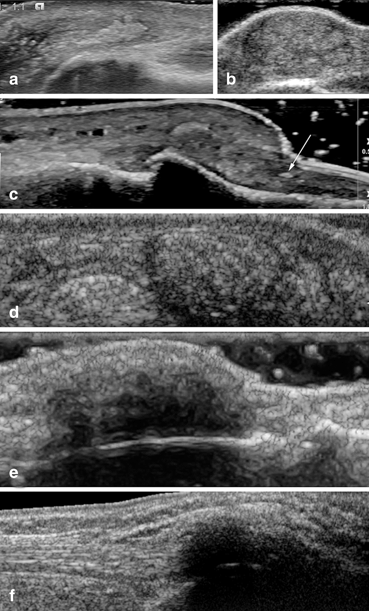

Fig. 7.7
Different aspects of the tophi gout: a soft tophus in the olecranon bursa; b pseudotumoral aspect of a soft tophus in the olecranian region; c soft tophi in the dorsal aspect of the distal interphalageal region. Note the extension of the deposits in the nail bed (arrow); d mixed tophi in the olecranian region; e mixed tophus over the calcaneus. The calcaneus bone contour is still visible; f hard tophus in the Achilles tendon. The posterior acoustic shadow covers the calcaneus bone contour
The most frequent characteristics of tophi in the olecranon bursa were: hyperechogenicity (91.7 %), poorly defined contours (88.6 %), multiple grouped nodules (85.6 %), and heterogeneity (68.6 %) [38].
Tendons and Ligaments
Tophi in tendon and ligaments were defined as heterogeneous hyperechoic (relative to tendon/ligament fibers) aggregates with poorly defined margins with or without areas with acoustic shadowing within and/or around the tendon or ligament, respectively. MSU crystal deposition in tendons was also defined as hyperechoic (relative to tendon fibers) linear bands within the tendon substance [13–20, 25, 31] (Fig. 7.8).
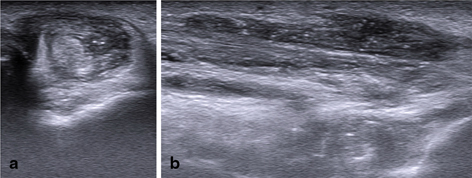

Fig. 7.8
Gout. Transversal (a) and longitudinal (b) scan of the peroneal tendons. The tendon sheath is filled with hypoechoic inhomogeneous material with hyperechoic spot
The literature reveals that the most frequently affected tendons and ligaments by tophi are the patellar ligament, the triceps tendon, the quadricipital tendon, under the lateral collateral ligament, the Achilles tendon and the anterior tibialis tendon, and plantar fascia [13, 15, 17, 20, 29, 30, 39, 40] (Fig. 7.9).
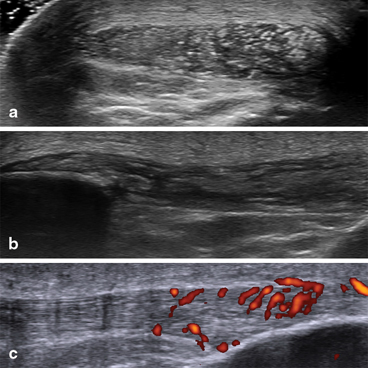

Fig. 7.9
Microdeposits of monosodium urate crystals and intra-tendinous tophi in the patellar tendon, associated with hypervascularization: a transversal scan; b longitudinal scan; c power Doppler examination
Naredo et al. found tendon abnormalities most frequently in the patellar tendon (60.4 %) and the triceps tendon (47.3 %), followed by the quadriceps tendon (38.5 %) and the Achilles tendon (34.1 %) [16].
Aggregation of MSU crystals at the insertion of the tendons or ligaments (enthesis) leading to gouty enthesopathy [17, 20].
Tophus Measurement and Assessment of Treatment Response by US
Eight methods of tophus measurement have been described in the literature: counting the total number of tophi, physical measurement using tape measure, physical measurement using Vernier calipers, digital photography, US , MRI, CT, and dual energy CT (DECT). US has an important role in monitoring tophus measurement in patients receiving ULT. The literature reports good sensitivity to change, associated with ULT [40] and good intra-observer correlation at follow-up [22], so tophi measurement could become an outcome measure for ULT in chronic gout [15, 25].
Tophus size can be directly measured using US calipers and can be reported as longest diameter and total volume. Diameters can range from less than 1 mm (microtophi) to centimeters. Decrease of tophus volume objectifies a treatment response. Ultrasound probes with three-dimensional capabilities may be used for an operator-independent measurement of volume [13, 14].
In a study by Perez et al., 68 % of tophi detected and measured with US showed significant reduction in tophus size in patients with an average serum urate < 6 mg/dl after 1 year of ULT (allopurinol and benzbromarone), compared with tophi where the urate remained > 6 mg/dl [7].
After treatment with pegloticase for 3 months, 22 % of subjects experienced complete resolution of the target tophus. After 6 months, 45 % experienced complete resolution [36].
Asymptomatic Hyperuricemia
The US examination should not be limited to the painful joints. The search for deposits of MSU crystals in patients suspected of gout should also be extended to asymptomatic joints previously involved in acute attacks [19, 30].
There is a wide spectrum of subclinical structural damage in both intra- and extra-articular structures in asymptomatic individuals induced by hyperuricemia [33].
Urate deposits in tendons and the synovium and the prevalence of patellar and Achilles enthesopathy occur more frequently in subjects with asymptomatic hyperuricemia than in asymptomatic, normouricaemic individuals [29, 33, 40].
Some authors have observed that the PD signal was even present in asymptomatic joints from gout patients and in asymptomatic hyperuricemia [26, 37].
Tophi were found in tendons (distal patellar tendon), synovium (knees and ankles), and other soft tissues in 34 % of patients with asymptomatic hyperuricemia [37].
Another published US study found significant number of patients with asymptomatic hyperuricemia and MSU crystals (joints with US-proved effusions were aspirated). DCS or “hyperechoic cloudy areas” were seen in 11/26 with 100 % sensitivity and 88 % specificity. [42].
Some author have suggested the need for use of the terms “subclinical gout” or “asymptomatic gout” instead of the old term “asymptomatic hyperuricemia” [21, 36, 39].
A study found DCS in 25 % of the MTP I and 17 % of the knees, erosions in 12 % of the MTP I’s and tophi/intra- and extra-articularly/in 18 % of hyperuricemic patient but in none of the normouricemic controls [21]. Another study found small tophaceous deposits in 34 % of patients, and an increased power Doppler signal in 23 % of them [26].
Urate deposition is present in more than half of gout or hyperuricemic patients who do not require ULT according to the European League Against Rheumatism (EULAR) recommendations [13].
These US findings open a new battlefront in the current debate about the need for treatment necessity of urate-lowering agents in asymptomatic patients with persistent hyperuricemia and indisputable US features of MSU microcrystal tissue deposition such as the DCS or the presence of tophi [33].
US-Guided Interventions
US is also the primary imaging modality used for needle guidance during diagnostic and therapeutic interventions.
US can help the clinician to localize synovial fluid in symptomatic or asymptomatic gout joints for aspiration [13, 25].
US can assist in the diagnosis of gout, allowing needle guidance for fluid aspiration for microscopic assessment of microcrystals [12].
In addition, US can guide aspiration of accessible extra-articular tophi for microscopic confirmation of the presence of microcrystals [16].
In conclusion, MSUS is a method of choice for diagnosis and management of gout and asymptomatic hyperuricemic patients.
Calcium Pyrophosphate Deposition Disease
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree