Location
Structure
Main findings
Gamuts
Shoulder
Long head bicipital tendon
Empty groove
Intraarticular portion of the long head of the biceps tendon is the most frequent location
Coracohumeral ligament could be torn alone or in association with supraspinatus tear
Two biceps tendons that really represent a longitudinal tear
The absence due to complete tendon tear
Tendinopathy
Hand
Flexor tendons
Empty anatomical site
Snapping sensation
Flexor contracture
Partial distal tears
Painful flexion with dynamic scanning due to associated trigger finger
Extensor carpi ulnaris tendon
Dislocation is normally volar side
There is no empty groove sign
Dynamic scan should include supination, ulnar deviation, and flexion movements
Dorsal subluxation directs to think about distal radius fracture
Ankle
Peroneal tendons
Always dislocate anteriorly
Partial or complete retinaculum tear
Intra-sheath peroneal tendon luxation
Longus and brevis peroneal tendons can reverse their anatomical position during dynamic scan
Peroneus quartus is a frequent accessory muscle in the ankle (22 %)
Look for avulsion near the retinaculum or malleolus
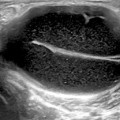
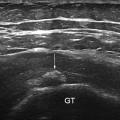
In the shoulder, the long head of the biceps tendon is the most representative lesion in association with a supraspinatus tear and coracohumeral ligament tear or lesion. The most frequent location is the intra-articular portion due to its curvilinear course and reflection over the humeral head. The sonographer should look for hypoechoic triangle that lies between the tendon and the medial border of the upper portion of the groove that in the case of disappearance, indirectly means subluxation [8], the demonstration of which requires dynamic external rotation (Fig. 11.1).
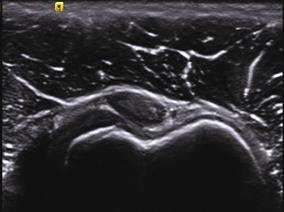

Fig. 11.1
Shallow bicipital groove and tendency of biceps tendon subluxation. Note the disappearance of the hypoechoic triangle that lies between the tendon and the medial border (left side of the figure) of the upper portion of the groove
In the hand, damage of the A2 and A4 annular pulleys in the flexor tendons are the most common sites. The ring and middle fingers are commonly involved. Excessive traction on the flexor tendons when the tendons are flexed causes anterior bowstringing which no longer lines against the bone plane; if this rupture is not diagnosed, flexion contractures can be present alongside other tendon lesions [9].
Other sites of lesions are the dorsal carpal bones between the carpometacarpal interline and the extensor tendons at the level of the radius or fingers [10]. Extensor compartments contribute widely to subluxation and wrist instability; sixth compartment is the most commonly involved due to its particular anatomy. The extensor carpi ulnaris tendon is normally held between a fibro-osseous sheath and instability may result in either subluxation or intermittent dislocation. Dynamic maneuver should be performed doing progressive supination of the forearm with ulnar deviation and flexion while the probe is in the short axis at the ulnar styloid [11, 12] .
Ankle pathology involves peroneal tendons in patients with chronic ankle sprains and sport injuries. Normally, laxity of the superior peroneal retinaculum, valgus, and accessory tendons are involved in the etiology; although it can be voluntary or could secondarily involve disruption of the superior peroneal retinaculum in various grades. Clinically, it is revealed with forced dorsiflexion and eversion of the foot, showing a palpable snapping or discomfort with pain. Ultrasound examination in the short axis should be dynamic to look for abnormal tendon movement over the malleolus or intra-sheath luxation [13] .
Tendinopathy: Tears
The damage produced in the collagen fibers of the tendon is usually referred to as tendinopathy . This term refers to specific changes in the appearance of the tendon, histological changes in the collagen and tendon disruption that makes collagen fibers thinner with the loss of the hierarchical structure with consequent wound and subsequent healing . With ultrasound, the primary findings are tendon thickening, heterogeneous hypoechogenicity, and the loss of parallel superficial and deep tendon borders. These first changes, referred to as tendinosis, can persist for months or even years, but if damage continues, it will lead to hypoxia and myxoid degeneration that cause ischemia and focal zones of hypovascularization, resulting in tendon rupture [14, 15].
Normally tears are not produced unless external and severe trauma or excessive loading forces involve the musculotendinous system. When a tendon tear appears, it typically does so at the insertion near the bone or at the myotendinous junction. Tears can be partial, complete, or intrasubstance, depending on the particular region or the mechanism that caused it [16–18] (Table 11.2.).
Table 11.2
Tendon tears
Mechanism | Tendon | Main findings | Gamuts |
|---|---|---|---|
Overuse damage | Supraspinatus tendon Long head of the biceps tendon Extensor and flexor tendons of the elbow Patellar tendon Achilles tendon | Tendinosis is focal or diffuse, poorly demarcated Double cortex appearance and cortical irregularities Spur formation in epicondyle, patella, tibia, and calcaneus Non-insertional paratenonitis with partial tears in Achilles tendon injury could be associated Bursal fluid often present | Compression with the probe to obliterate space when there is fluid with the tear Intrasubstance tears are hypoechoic areas within the tendon not related to articular or bursal surfaces Biceps tendon, complete tears, tracing the muscle belly in the arm differentiates from dislocation Tendon calcification could be present Ultrasound scanning in the knee with slight flexion might be necessary In Achilles tendon distal tears, plantaris tendon insertional portion could be intact |
Excessive frictional forces Steroid treatment | All regions | Tendinosis Paratenonitis Tears | Avoid anisotrophy Initial stages only focal regions of low echogenicity Steroids can be seen as intrasubstance hyperechoic spots depending on the time of injection |
Inflammatory and metabolic systemic diseases | Quadriceps tendon Patellar tendon Extensor and flexor digitorum tendons Posterior tibial tendon | Bursitis could be present Enlargement or thickening of the tendons Loss of definition of tendon margins Anechoic ring at the extensor retinaculum or the malleolus | Doppler signal could be present in active diseases |
Partial tears appear as a region devoid of tendon fibers while complete tears cause tendon disruption with visible tendon stumps. Dynamic imaging shows approximation (Fig. 11.2).
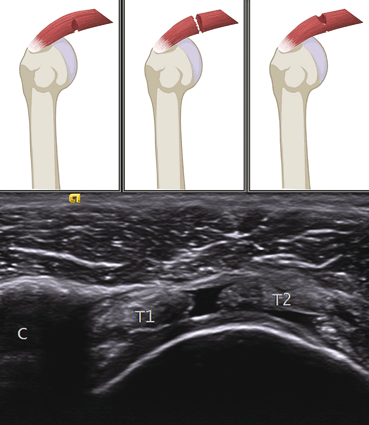

Fig. 11.2
Supraspinatus tendon tears. The drawing shows different examples of partial and complete tears. The ultrasonography image shows complete tear with tendon edges (T1 and T2) retraction. C = coracoid process. Note the anechoic fluid replacing the tendon. Also of relevance is the double cortex sign (cartilage interface sign)
Paratendinitis
Paratendinitis refers to an inflammatory process that involves the vascular paratenon which surrounds the patellar and Achilles tendons mainly . It represents a continuous inflammatory process that causes high-grade hypoxic tendinosis. Ultrasound features include thickening of the paratenon, irregular tendon margin, and fluid, with or without color/power Doppler. Bursitis is frequently associated and reflects irritation or local overload and work stress. Soleus and gastrocnemius muscle injuries could be present in association with Achilles paratendinitis (Fig. 11.3).
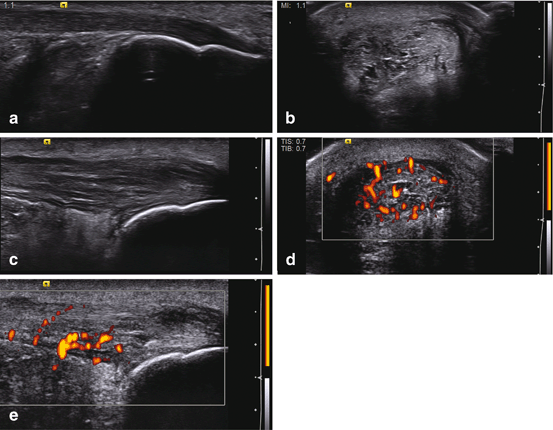

Fig. 11.3
Achilles tendinitis and paratendinitis. (a) Normal Achilles tendon in long axis. (b) Short axis. (c) Long-axis gray scale with increased thickness, hypoechoic changes, fibril separation, edema, and loss of normal tendon boundary. (d) Short axis. (e) Long-axis power Doppler for the same findings with positive signal in and around the tendon
Tenosynovitis
In tendons with a synovial sheath, inflammation is secondary to mechanical factors (repetitive microtrauma, overuse, or osseous friction), foreign bodies, infection, tumors, or inflammatory entities (e.g., rheumatoid arthritis) . Acutely, inflammation is characterized by fluid encircling the tendon forming a ring or halo around it; chronic disease causes tendon sheath thickening that may cause tendon entrapment and/or thicker retinacula or pulley (e.g., de Quervain’s disease, trigger finger of flexor and extensor tendons, peroneal entrapment) [9, 19] (Figs. 11.4, 11.5).
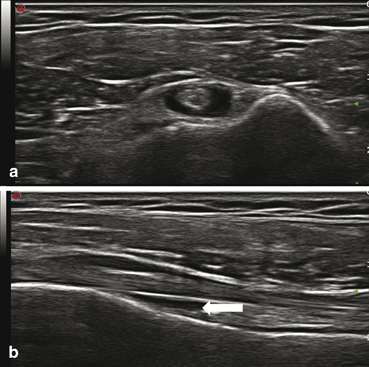
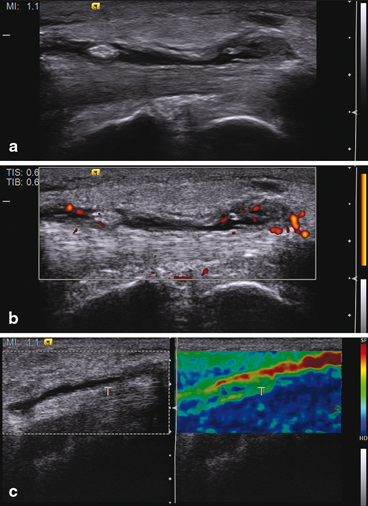

Fig. 11.4
Tenosynovitis of the biceps tendon. (a) Short- and (b) in long-axis views demonstrate effusion (arrow)

Fig. 11.5
Tenosynovitis in different ultrasonographic modalities. (a) In gray scale showing anechoic effusion together with echogenic synovial hypertrophy. (b) With power Doppler confirming synovial hypertrophy as well as activity. (c) With elastography in which synovial effusion appears softer than the tendon
In the hand, effusion is easy to identify when it is proximal to the metacarpal head. In the fingers, effusion assumes a lobulated appearance and creates a discontinuous array of the pulleys. In infection processes, there is no characteristic image but tenosynovitis seems to be more hyperechoic and the subcutaneous tissue appears hyperechoic with thickening .
Special Conditions: Trigger Finger
Trigger finger is the common term for stenosing tenosynovitis, usually affecting the flexor tendons in the thumb and index finger and involves A1 pulley pathology, although it can affect other fingers and other pulleys as well. It is a painful condition in which the tendon locks when flexed and is difficult to extend due to inflammation of its tendon sheath, provoking a click sound when the tendon passes through it. The normal pulley, visualized by ultrasound, appears as a hypoechoic band superficial to the flexor tendon sheath. In the short axis, lateral expansion looks hypoechoic causing an anisotropic artifact that could be reduced by tilting the probe. In trigger finger, the pulley looks like a diffuse hypoechoic thickened band at the level of the metacarpophalangeal (MCP) joint which might exhibit Doppler signal. Dynamic scanning allows for the detection of changes in the shape of the synovial sheath and tendon pathology such as peritendinous effusion, tendinosis, or tenosynovitis. Additionally, ultrasound allows for the guidance of local infiltration therapy [19, 20].
Enthesopathy (Noninflammatory)
According to the Outcome Measures in Rheumatoid Arthritis Clinical Trials’ (OMERACT) definition, enthesopathy is an “abnormally hypoechoic (loss of normal fibrillar architecture) and/or thickened tendon or ligament at its bony attachment (may occasionally contain hyperechoic foci consistent with calcification), seen in 2 perpendicular planes that may exhibit Doppler signal and/or bony changes including enthesophytes, erosions, or irregularity” [21] .
In non inflammatory lesions, the most common injury mechanism is sport related or direct trauma. Affected regions are the elbow, hand, knee, and ankle (Fig. 11.6).
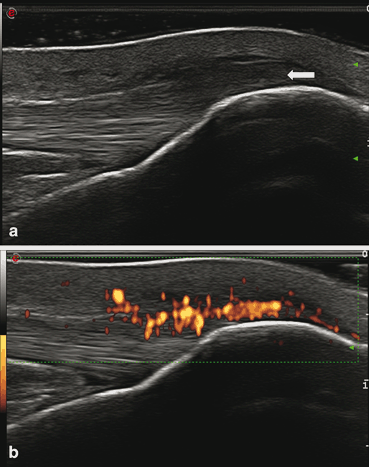

Fig. 11.6
Enthesopathy of the distal patellar tendon in long axis. (a) Gray scale shows increased thickness and hypoechoic changes (arrow). (b) Power Doppler signal in the same hypoechoic area
Surgical Changes in Tendon
Surgical treatment in any tendon has the main objective of restoring function—indications vary but the appearance depends on the various processes tendons undergo to heal. These conditions are related to histological healing , complete tendon fiber restitution, and clinical and functional evolution [22]. Histological changes are related to cell infiltration and neovascularization that could be visualized with color Doppler signal present at the site of healing or at the most painful site when clinical exam is performed. With the use of novel techniques such as contrast-enhanced ultrasound, it is possible to visualize the increased vascular flow in the areas of interest, where Doppler might fail [23].
Depending on the history of the tendon injury, its location, time between injury and surgery, surgical technique performed, postoperative complications and course are the findings we have to look for in the tendon. It is important to note that sutures, surgical wires, or intratendinous surgical material could be found during the ultrasound exam. We also have to remember that, depending on the time after surgery, the tendon can present with different lesions as shown in Table 11.3 (Fig. 11.7).
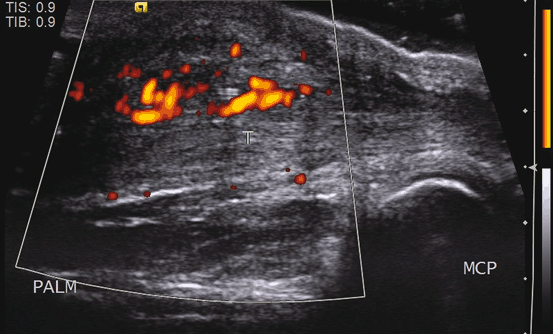

Fig. 11.7
Postoperative tendon (T). The flexor tendon is thickened and surrounded by thick echogenic tissue that exhibits Doppler signal, representing adhesions
Table 11.3
Characteristics of operated tendons
Characteristics | Ultrasound features | Description |
|---|---|---|
Morphology | Thickness and width | Loss of the normal pattern Thicker the first 3 months, irreversible Tendon stumps cause focal thickness or callus Re-rupture: Thinner when re-ruptured Loss of tendon pattern and/or continuity of the tendon Anechoic image between the opposite parts of the tear and/or hematoma |
Contour | Circumferential, hypoechoic, peritendinous area after surgical repair up to 3 months Hypoechoic–anechoic halo surrounding the repaired tendon | |
Continuity | Anechoic images in the repaired zone A re-rupture could appear in the repair zone | |
Structure | Internal | Loss of fibrillar pattern Heterogeneous structure Hypoechoic areas surrounding the stitches and metal devices if present |
Vascularization | Intratendinous Peritendinous | No vascularization immediate to surgeryIntrasubstance the first month, small or few Doppler spotsHypervascularization next 3 months No Doppler after 6 months In peritendinous areas could be no Doppler signal at any time |
Dynamic scan | Active Passive | Immediate after surgery, there is no movement at all The first 6 months, reduction of movement is observed and depends on the type of surgery performed and the repaired surface |
Other | Associated lesion | Synovitis Enthesitis Cortical irregularities Intratendinous ossification or calcification Surgical artifacts: sutures, surgical wires, or intratendinous surgical material Adhesions |
Elastography | Soft Heterogeneous | |
Contrast-enhanced ultrasound | Displayed the maximum intensity of vascularization in suture anchor and/or the tendon peripheral structures (bursae, muscle, peripheral bursal fat stripe) after surgery |
It is important to note that in tendons with tendon sheaths, the main lesion is tenosynovitis; in tendons without it, lesions such as tendinopathy, tendonitis, and paratendinitis could be present. Tendon tears after surgery deserve a special mention—these lesions can appear in the repaired tendon or within the substance around the lesion of importance and needs to be confirmed in longitudinal and transverse views because anechoic spots do not necessarily mean rupture and could be part of the healing process. In the operated tendon, complete tears can occur in the first month and it is mandatory to look at the other areas, probably away from the injury site and hematoma. Structures such as retinaculum, muscle, and ligaments at the peripheral surface surrounding the affected tendon can be damaged [24, 25].
In general, when a tendon is damaged, it loses its mechanical properties—ultrasound gives information of the lesion in many aspects, both in gray scale and Doppler, but new techniques such as elastography improves the diagnostic capability of conventional ultrasound. This technique involves manual compression of the tendon to generate tissue deformations. The strain distribution of the involved region could be compared before and after this tissue deformation (axial elastography). While this technique could be highly operator dependent and erratic regarding reproducibility, the most recent shear elastography, which depends on the intrinsic tissue elasticity shown, seems promising (Fig. 11.8).
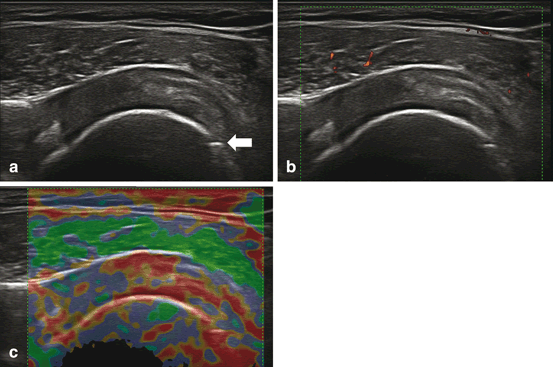

Fig. 11.8
(a) Supraspinatus tendon 2 years postsurgical repairing, the image is in longitudinal view and gray scale exhibit as hypoechoic blurred structure. (b) Doppler signal is located in the muscle. (c) Elastography shows a marked red area in the zone of the repair (soft); an anchor is located distally (arrow).
The most studied tendon with elastography has been the Achilles followed by the common extensor tendon in the elbow. In the Achilles, the normal tendon is harder and more homogeneous when compared with surgically repaired tendons which appear softer and more heterogeneous. Although elastography is still in the process of being validated for use in rheumatology and musculoskeletal specialties, the technique has proven useful in demonstrating changes in tendon architecture and is a good method to improve the assessment of other structures as well [26].
Muscle
Skeletal muscle is the largest tissue in the human body, composed of parallel fibers surrounded by connective tissue that is organized into group of bundles called fascicles which are in turn surrounded by tissue called the perimysium and finally enclosed in the epimysium . Due to its function and anatomic position, these fibers are susceptible to lesions. Ultrasound appearance of the muscle is affected by many factors including angulation of the ultrasound beam. Sport is the most common cause of injury [27] .
Classification of muscle lesions is acute and chronic. Acute lesions are tears, contusions, and lacerations, whereas chronic lesions are scars, fibrosis, and myositis ossificans:
1.
Strain, tears, and lacerations commonly result from over elongation; signs are localized and cause tender points that increase with active movements [28].
a)
The main objective of ultrasound in acute lesions is to determine the severity and extent of the lesion. There are several systems for classifying lesions though none of them are accurate or globally accepted. Ultrasound features can however be graded into :
Grade 0: minimal muscle changes that could be seen as normal in ultrasound.
Grade I: focal or generalized hyperechoic areas located within a bundle, represents ≤ 5 % of the total muscular area affected.
Grade II: loss of muscle or perimysial striation or discontinuity of the fibers with vascularization; loss of echogenicity and a hyperechoic halo surrounding the myotendinous junction or hematoma. Lesion involves more than 5 % of the muscle area. Dynamic ultrasound could reveal the bell clap sign (Fig. 11.9).
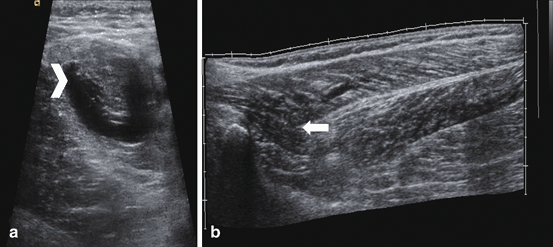
Fig. 11.9
Muscle showing the central aspect of the muscle tear (arrow head) in short axis, and B grade II tear (arrow) in long axis
Grade III: complete disruption of the muscle and loss of longitudinal fiber integrity; associated with hematoma and/or muscle retraction (Fig. 11.10).
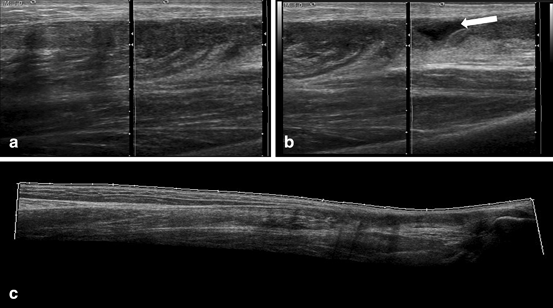
Fig. 11.10
(a) Medial gastrocnemius muscle long axis, in gray scale with muscle grade II tear. (b) double screen view in long axis of the medial soleus muscle with a hematoma (arrow), and (c) panoramic view of the Achilles tendon and soleus muscle in which tendon rupture is shown and muscle tear is depicted
b)
Hematoma healing starts immediately after the lesion: firstly, the hematoma undergoes a process of liquefaction, with subsequent resorption during which showing a very heterogeneous appearance with areas of reflective material or fibrous septae . Over time, these areas disappear and muscle fibers reorganize and appear normal—complete recovery can take weeks or months. With repeated trauma, granulomatous tissue forms and a scar appears. If the hematoma fails to resolve, a cyst forms and a discrete area devoid of reflectivity with acoustic shadowing is seen [29] .
c)
Contusion is the lesion that results from direct blunt impact and is most frequently associated with sport . Muscle contusion represents a capillary and muscle fiber disruption with microhemorrhage, dissecting fibers and leading to inflammation—ultrasound shows mild or focal muscle swelling against the background of the undamaged muscle tissue, secondary to hematoma in the first 24–48 h that normally resolves leaving no permanent damage. Over the next 2–3 days, collections become hypo- or anechoic increasing in echogenicity as time passes. After few weeks, the hematoma organizes and focal scar tissue develops if the contusion is large [30] .
d)
Acute compartmental syndrome arises following the trauma due to hematoma formation and muscle swelling—ultrasound can be helpful in identifying focal collections which, if drained, can decompress the compartment .
2.
Chronic muscle injury is the result of abnormal healing or extended lesions as a consequence of untreated acute lesions. The main chronic lesions are:
a)
Scars that appear as linear or irregular echogenic structures surrounded by hypoechoic zones that are usually found at the fascial interface or myotendinous junction .
b)
Muscle herniation results from fascial defects that allow extra compartmental herniation , commonly in the lower limb, which become prominent with muscle contraction. Ultrasonographically, there is normal muscle through the focal epimysial defect; longitudinal images show perimysium bowed into the defect. If the defect is large, bell clap sign is observed with active motion.
c)
Atrophy is related to chronic inflammation; fatty infiltration and decreased muscle fibers result with an increased echogenicity of the muscle involved .
d)
Myositis ossificans is a benign non-neoplastic condition, often seen in young people, following muscle trauma and is defined as heterotopic bone or cartilage formation in or adjacent to muscle . There are four types of the condition but the most common is post-traumatic. Phase changes can be detected by ultrasound; during early phase (precalcified), ultrasound can show a vascular solid mass with Doppler signal present at the periphery, but caution and vigilance are required because this image can also be seen in sarcoma. Late phase (calcified) appears as a hypoechoic or heterogeneous mass with calcification, with a vascular rim in the central zone demonstrating enhanced Doppler signal. Progressively, Doppler disappears and an acoustic shadowing appears due to peripheral ossification [31] .