Malignant
Nonmalignant
Primary endoluminal carcinoma
Lymphadenopathy
Bronchogenic
Sarcoidosis
Adenoid cystic
Infectious (i.e., tuberculosis)
Mucoepidermoid
Vascular
Carcinoid
Sling
Metastatic carcinoma to the airway
Cartilage
Bronchogenic
Relapsing polychondritis
Renal cell
Granulation tissue from:
Breast
Endotracheal tubes
Thyroid
Tracheostomy tubes
Colon
Airway stents
Sarcoma
Foreign bodies
Melanoma
Surgical anastomosis
Laryngeal carcinoma
Granulomatosis with polyangiitis
Esophageal carcinoma
Pseudotumor
Mediastinal tumors
Hamartomas
Thymus
Amyloid
Thyroid
Papillomatosis
Germ cell
Hyperdynamic
Lymphadenopathy
Tracheomalacia
Associated with any of the above malignancies
Bronchomalacia
Lymphoma
Webs
Tuberculosis
Sarcoidosis
Goiter
Mucus plug
Vocal cord paralysis
Epiglottitis
Blood clot
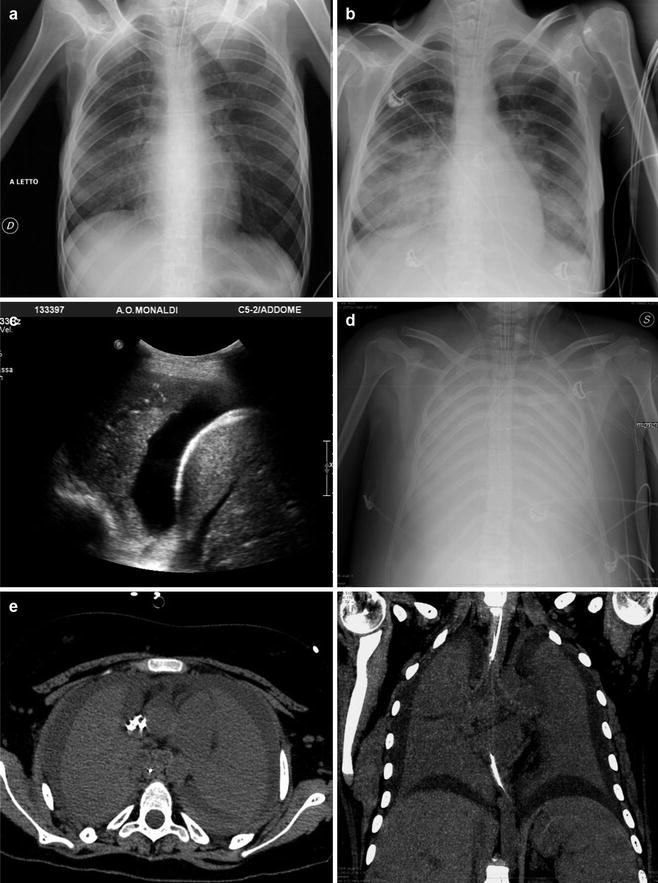
Fig. 1
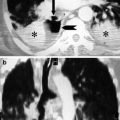
Acute complete lower airway obstruction by rapid and severe course of toxic shock syndrome (TSS) in MRSA fatal progressive pneumonia. A 26-year-old menstruating woman with high fever, rash, hypotension, and rapidly evolving multiorgan failure is immediately transferred from ED into the ICU. (a) CXR at the admission in the ICU shows right lower lobe opacity. (b) Follow-up CXR 24 h later shows a new contralateral basal opacity. (c) Bedside longitudinal right LUS shows low right lobe complete consolidation associated to pleural effusion. (d) Patient in extracorporeal membrane oxygenation (ECMO). Twenty-four hours later, follow-up CXR shows completely opacified lungs. (e, f) Unenhanced MDCT axial and coronal MIP reconstructions show bilateral complete lung consolidation associated to pleural effusion. Note hypodense material that fills large airways
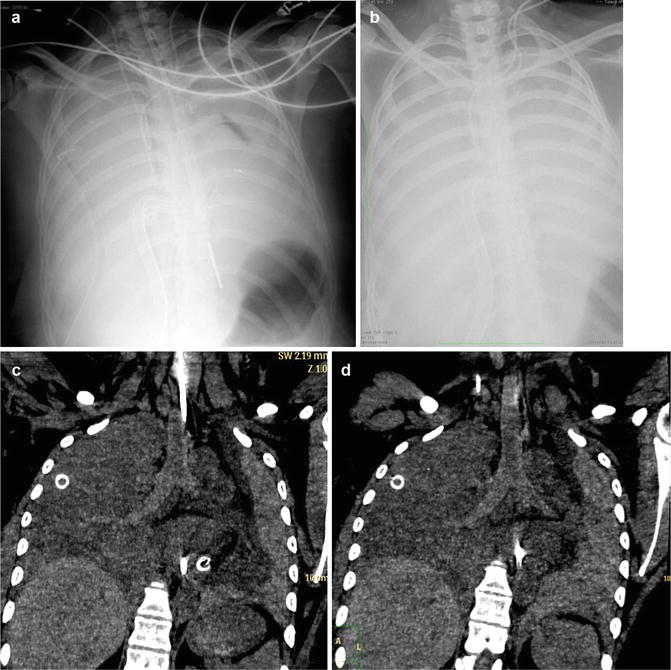
Fig. 2
Acute lower complete airway obstruction in a 16-year-old male with bleeding diathesis by treatment with anticoagulant therapy, abrupt massive postnasal bleeding, and airway hemorrhage by an highly vascularized bleeding nasopharyngeal angiofibroma.(a) CXR after nasogastric tube and right pleural drainages placement shows subtotal opacified lungs. (b) The patient was immediately transferred from ED into the ICU and CXR showed total opacified lungs.(c, d) Emergent unenhanced MDCT coronal reconstructions show hyperdense blood material that fills airways and the bloody hyperdensity mainly in the left lung (compare with Fig. 1). After nasopharyngolaryngoscopy, this patient was treated by emergent preoperative embolization. Juvenile nasopharyngeal angiofibroma is a benign, highly vascular hamartoma that arises from the nasopharynx almost exclusively in adolescent males
1.2 Imaging
Patients with an acute upper airway complaint need to have their breathing issues addressed before being transferred to the radiology suite for imaging. Intubation should be considered before any examination that requires patients to be supine (i.e., CT and MR imaging), especially if there is any suspicion of anaphylaxis or other difficulties in maintaining oxygen saturation levels. Portable anteroposterior (AP) chest radiography (CXR), complementary sonography (lung ultrasonography, LUS), and MDCT scanning of the neck and chest are the most effective initial emergent imaging studies to assess the location and extent of the airway obstruction. For patients who can tolerate lying flat for the study, MDCT provides the anatomic detail that permits planning of therapy. The study is assessed for location of primary tumor/mass, the degree of intrinsic versus extrinsic compression, and the length of the stricture. Furthermore, the relationship of the narrowing of the trachea relative to the larynx or the carina is readily assessed. For bronchial lesions, the extent of postobstructive atelectasis or infection is readily observed.
Flexible bronchoscopy provides a definitive view of the intraluminal disease but should be performed with preparation for management of the airway obstruction with laser, rigid bronchoscopy, or stent placement.
1.3 Pulmonary Parenchymal Abnormalities
Atelectasis, aspiration, airspace opacification or consolidations, air trapping and hyperinflation, hydrostatic pulmonary edema, and noncardiogenic pulmonary edema all present as pathological findings on CXR, LUS, and MDCT. Although it is often difficult, and sometimes impossible, to distinguish between these entities, certain radiographic features can aid in their diagnoses.
1.3.1 Obstructive Lung Diseases
Acute Exacerbation in Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is defined as incompletely reversible expiratory airflow obstruction, likely caused by exposure to noxious inhaled particles (Rabe et al. 2007). The airflow limitation that underlies functional obstruction is usually progressive and associated with an abnormal inflammatory response of the lung. What is clinically called COPD reflects a complex syndrome encompassing potentially overlapping diseases such as pulmonary emphysema, chronic bronchitis, and small airway disease. Acute exacerbation of COPD is defined “as an event characterized by a change in the patient’s baseline symptoms that is beyond normal day-to-day variations” is acute in onset and may warrant a change in regular medication in a patient with underlying COPD (Table 2) (Siafakas et al. 2004; Rabe et al. 2007). Three COPD phenotypes were identified based on morphologic CT changes and clinical features of COPD: phenotype A, characterized by no or minimal emphysema with or without bronchial wall thickening; phenotype E, characterized by emphysema without bronchial wall thickening; and phenotype M, characterized by emphysema with bronchial wall thickening (Fujimoto et al. 2006).
Table 2
Summary of diseases that provoke, precipitate, or mimic an acute COPD exacerbation (Siafakas et al. 2004)
Parenchymal diseases | Pneumonia and their complications; complicated bullae |
Airway diseases | Bronchial carcinoma; tracheobronchial tree infections, common pollutant |
Cardiac diseases | Congestive cardiac failure, right heart failure |
Lung vessels | Pulmonary hypertension, acute embolism, hemoptysis |
Pleura | Pleural effusion, pneumothorax |
Muscles | Muscular wasting |
Mediastinum | Pneumomediastinum |
Acute Exacerbation in Asthma
Asthma is characterized by all of the following: (1) airway obstruction that is usually reversible, (2) chronic airway inflammation, and (3) nonspecific airway hyperreactivity.
The CXR features of asthma are not particularly specific, but the most common abnormality is bronchial wall thickening, with hyperinflation the second most common (identified up to 24 % though less reliable) finding (Lynch 1998). In patients with asthma, CT is indicated to identify suspected complications, particularly allergic bronchopulmonary aspergillosis, and mimics of asthma such as hypersensitivity pneumonitis. When it is performed, CT is helpful for evaluating the extent of airway thickening (remodeling: airway walls are thicker than healthy individuals and the degree of airway wall thickness directly correlates with the severity of airflow obstruction and clinical disease) (Aysola et al. 2008), bronchial dilation, and expiratory air trapping. Air trapping is usually defined as the percentage of the lung less than 850 HU on expiratory CT, and those individuals with an air-trapping percentage above the median value of 9.66 % defined as having an air-trapping phenotype. Bronchial dilation, or bronchiectasis (defined as a bronchus with a larger diameter than the internal diameter of the adjacent pulmonary artery), is more prevalent in asthmatic patients than in healthy subjects. Acute complications of asthma may include pneumothorax, pneumomediastinum (and rarely pneumopericardium, pneumoperitoneum, pneumoretroperitoneum, pneumorrhachis, and even subdural emphysema), mucus impaction with or without atelectasis, and pneumonia (Woods and Lynch 2009).
1.3.2 Consolidations or Airspace Opacifications or Alveolar Opacity or Alveolar Pattern
Consolidation is the most common cause of pulmonary opacities in the acute airway obstruction population. In contrast to ground-glass opacities (GGO), consolidation obscures underlying vascular structures and frequently is associated with absence of volume loss and air bronchograms by opacification of lung acini with little or no involvement of conducting airways. By definition, diseases that produce consolidation are characterized by a replacement of alveolar air by fluid, cells, tissue, or some other substances (Hansell et al. 2008). If acute, consolidation is most commonly caused by pulmonary edema (of both cardiogenic and noncardiogenic causes), pulmonary hemorrhage, acute eosinophilic pneumonia, acute extrinsic allergic alveolitis, radiation pneumonitis, pulmonary infarction, or infectious pneumonia (Table 3).
Table 3
(a) Diseases recognized as causing alveolar opacity or pattern
Transudate |
Hemodynamic pulmonary edemaa |
Non-hemodynamic pulmonary edema |
Diffuse alveolar damage (ARDS)a |
Exudate |
Infective pneumoniaa |
Eosinophilic pneumonia |
Lipoid pneumonia |
Hemorrhage |
Diffuse alveolar hemorrhage (DAH) |
Severe cardiac failurea |
Pulmonary infarctiona |
Emboli a |
Thromboembolisma |
Fat embolism |
Amniotic fluid embolism |
Bone marrow embolism |
Infiltration |
Adenocarcinoma ex-bronchoalveolar cell carcinoma |
Lymphoma |
Miscellaneous |
Pulmonary alveolar proteinosis |
Acute extrinsic allergic alveolitis |
Sarcoidosis |
Radiation pneumonitis |
Table 3
(b) Imaging signs of acute obstruction of airways by alveolar disease
1. Acinar or peribronchiolar nodules |
2. Air alveologram and bronchiologram |
3. Air bronchogram |
4. Butterfly or “bat’s wing” distribution |
5. Coalescence (early) |
6. Fluffy, ill-defined margins |
7. Perihilar, diffuse, segmental, or lobar distribution |
8. Present soon after onset of symptoms; rapid change |
1.3.3 Thoracic Oncologic Emergencies
Oncologic patients often experience emergent complications that are either direct result of the underlying malignancy or an indirect result related to the therapy (Table 4).
Table 4
Thoracic oncologic direct and indirect complications
Oncologic direct complications | |
Mass effect and airway compromise | Airway occlusion |
Tracheo- or broncho-esophageal fistula | |
Mass effect and vascular invasion | SVC obstruction, SVC syndrome |
Mass effect and invasion of cardiac chambers | Pericardial effusion and cardiac tamponade |
Tumor extension into the cardiac chambers | |
Pleural involvement | Massive pleural effusion, hemothorax, pneumothorax |
Chylothorax | |
Neovascularity | Massive hemorrhage, pseudoaneurysm, or AV fistula |
Systemic manifestations | Massive pulmonary embolism, disseminated intravascular coagulation, opportunistic infections |
Oncologic indirect complications | |
Postoperative or post-procedural | Pneumothorax, massive hemorrhage, hemothorax, complications of vascular injury, bronchopleural fistula |
Postradiation complications | Airway edema and obstruction |
Radiation pneumonitis, fistula formation | |
Chemotherapy-related complications | Spontaneous pneumothorax, immunosuppression and opportunistic infections, fistula formation |
Airway obstruction complicates approximately 20–30 % of patients with lung cancer, and large masses that compress the central airways may result in postobstructive atelectasis or pneumonia and airway compromise. Rapidly growing tracheal, mediastinal, and hilar masses are most common causes (Fig. 3) (Quint 2009; Guimaraes et al. 2014). Knowledge of airway narrowing is important before attempting percutaneous and surgical biopsy because it may lead to collapse of residual normal lung and sudden respiratory decompensation (McCurdy and Shanholtz 2012). Muscle relaxant drugs used during anesthesia may precipitate dangerous respiratory compromise in patients with pre-existing airway compression by tumor owing to loss of chest wall tone.
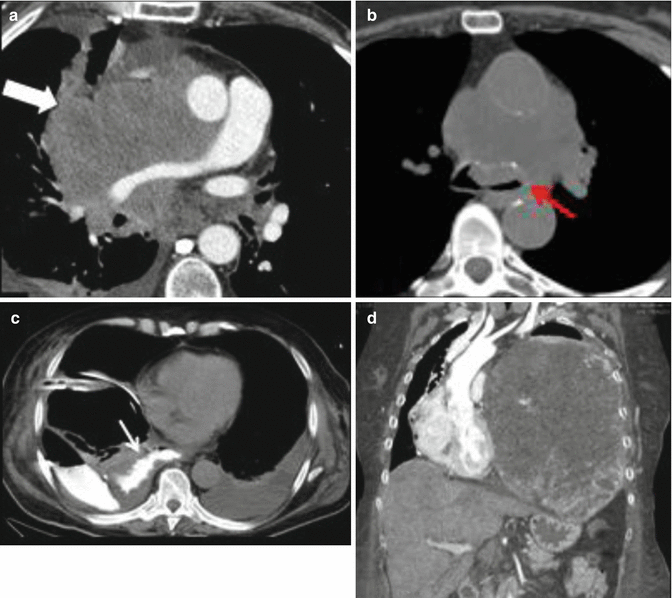

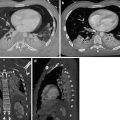
Fig. 3
MDCT of thoracic oncologic emergencies. (a) Lung small-cell carcinoma extending into the mediastinum with SVC syndrome (white arrow). (b) A soft tissue hilar mass with carinal and left bronchial extension (red arrow) in a known non-small cell carcinoma in a patient with acute breathlessness. (c) Esophageal carcinoma ruptured in right lung parenchyma and with esophago-pleural fistula (white arrow). (d) Mediastinal chondrosarcoma with rightward mediastinal shift and acute airway obstruction
1.3.4 Noncardiogenic Pulmonary Edema (NCPE)
Lung edema includes (1) hydrostatic edema caused by increased capillary pressure or decreased oncotic pressure, (2) ARDS (permeability edema caused by DAD resulting in interstitial and alveolar fluid accumulation), (3) permeability edema without alveolar damage (such as heroin-induced edema, edema following cytokine administration, and high-altitude edema) primarily resulting in interstitial fluid accumulation, and (4) mixed forms of hydrostatic and permeability edema (such as neurogenic edema, reperfusion and re-expansion edema, edema following lung transplantation, etc.)
1.3.5 High-Altitude Pulmonary Edema (HAPE)
High-altitude illness includes the cerebral syndromes of acute mountain sickness (AMS), high-altitude cerebral edema (HACE), and the pulmonary syndrome of high-altitude pulmonary edema (HAPE), recognized as an alveolar fluid accumulation in unacclimatized individuals by Houston in 1960 and attributed solely to high-altitude exposure (Houston 1960); it is mainly seen in children or young adults who rapidly ascend to heights of 2000–3500 m or greater and typically occurs 3–48 h after ascent, with the hallmark symptom being dyspnea at rest, often associated to dry or with frothy pink sputum production cough and restlessness. Approximately 50 % of patients with HAPE also show symptoms of AMS/HACE, but these two forms of altitude illness can exist independently of each other (Bartsch et al. 2005). The mechanism remains unclear: HAPE is believed to be a form of noncardiogenic pulmonary edema without alveolar damage caused by exposure to the low pO2 found at high altitudes. Hypoxia leads to uneven areas of vasoconstriction within the pulmonary vascular bed, resulting in a pattern of patchy overperfusion (Swenson et al. 2002); these foci of high vascular pressure lead to localized violation of the capillary endothelium, causing fluid extravasation. When available, CXR features initially are heterogeneous peripheral alveolar patchy, fluffy infiltrates progressing eventually to widespread, confluent airspace involvement more severe in lung bases, but the pattern of distribution is usually unpredictable. Portable lung ultrasonography provides a more feasible imaging option in remote locations and shows progressively larger numbers of extravascular fluid comet tails in patients ascending to altitude and suffering from HAPE (Fagenholz et al. 2007; Pratali et al. 2010). The most modifiable risk factor for the development of HAPE is the rate of ascent to high altitude; the mainstay of therapy increases alveolar and arterial oxygen by descent or with supplemental oxygen. HAPE is a potentially life-threatening illness that is completely preventable; as the popularity of trekking and climbing grows among the general population, physicians will be increasingly relied on as a source of education, prophylaxis, and treatment, making a working familiarity with altitude-induced illnesses such as HAPE an indispensable tool for emergency physicians.
1.3.6 Neurogenic Pulmonary Edema (NPE)
A number of intracranial conditions, including head trauma, hemorrhage (usually subarachnoid), tumors, and encephalitis, can be associated with acute pulmonary edema. It can occur within a minute to hours following a neurologic insult and usually resolves within 72 h. Patients may present with varying degrees of dyspnea, tachypnea, and cyanosis shortly after suffering the brain insult. The onset of symptoms occurs in <4 h in the majority and death in 10 %. The mechanism is unclear: it has been suggested that a sudden burst of neural activity stimulates the sympathetic nervous system and an adrenergic response leading to increased extravascular lung water, increased pulmonary hydrostatic pressure, and increased lung capillary permeability (Tan and Lai 2007). The CXR picture is indistinguishable from that of cardiogenic PE (nonspecific, bilateral, rather homogeneous airspace consolidative appearances with an apical predominance are present in about half of cases) except that the heart is not enlarged and it can take up to 24 h to be apparent on imaging (Fig. 4). Radiologic findings in neurogenic pulmonary edema also disappear within 1–2 days, thereby confirming the absence of any associated DAD (Gluecker et al. 1999).
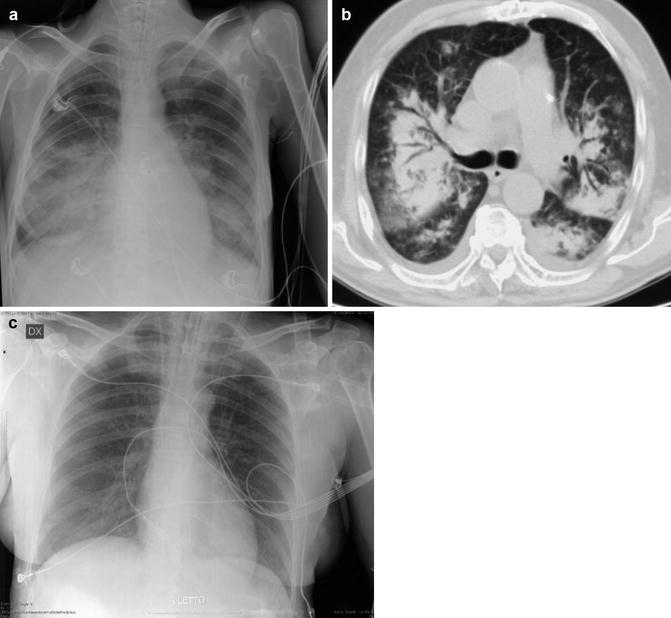

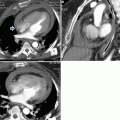
Fig. 4
Neurogenic pulmonary edema. (a) Anteroposterior bedside CXR view shows bilateral alveolar opacities in a 54-year-old woman with shortness of breath and subarachnoid hemorrhage, who developed neurogenic pulmonary edema. (b) MDCT axial scan shows diffuse bilateral and symmetric patchy ground-glass and consolidative opacities sparing the lung periphery. (c) Follow-up CXR 3 days later shows normal findings
1.3.7 Re-expansion Edema (REPE)
The condition occurs often in young patients in the setting of rapid expansion of a collapsed lung, with acute-onset shortness of breath usually occurring within hours of re-expansion. The onset of pulmonary edema can be delayed by up to 24 h in some cases (Echevarria et al. 2008). It occurs following approximately 1 % of pneumothorax re-expansions or thoracentesis procedures (mostly if large-volume drainage >3 l). Although the exact mechanism of REPE has not been identified, it appears to be caused by multiple mechanisms. Increased capillary permeability due to hypoxic injury, reperfusion injury with release of toxic oxygen free radicals, and surfactant depletion are all thought to play a major role. Furthermore, a delay in lymphatic return by stasis during prolonged collapse and bronchial occlusion may also partly account for the development of REPE. It can progress for 24–48 h and usually shows slow resolution over 5–7 days. REPE may lead to severe hypoxemia, which will increase lung damage and may result in extensive adult respiratory distress syndrome (ARDS) and multiorgan failure. CXR findings are alveolar opacities usually unilateral in those portions of the lung that were previously collapsed; rarely edema can develop in the contralateral lung (Baik et al. 2010). Thin-section MDCT findings of REPE are peripheral patchy areas of GGO frequently combined with consolidation as well as interlobular septal and intralobular interstitial thickening (Fig. 5). The British Thoracic Society guidelines suggest that less than 1.5 l of pleural fluid be drained at a time (BTS Pleural Disease Guideline 2010). Drainage catheters can be intermittently plugged to prevent rapid lung re-expansion. Rapid re-expansion of pneumothoraces is less easily controlled and caution should be taken to avoid high negative intrapleural pressures.
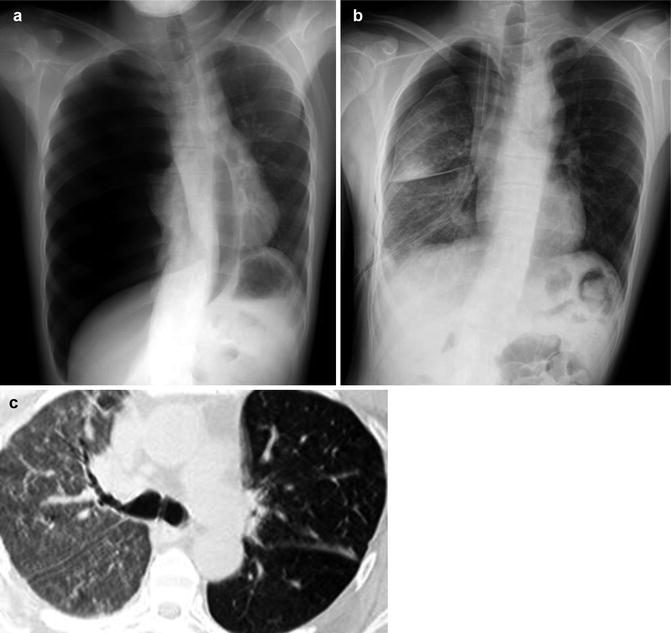

Fig. 5
Re-expansion pulmonary edema. (a, b) Standard erect PA CXR shows right lung re-expansion pulmonary edema following a spontaneous pneumothorax drainage. (c) Thin-section low-dose MDCT scan shows unilateral diffuse ground-glass opacity
1.3.8 Atelectasis
Atelectasis or collapse (Hansell et al. 2008), a decrease in lung volume accompanied by increased opacity (CXR) or attenuation (CT scan) in the affected part of the lung, is a common cause of pulmonary opacities in the acute airway obstruction population. One of the commonest mechanisms is resorption of air distal to airway obstruction (e.g., an acutely complicated endobronchial neoplasm or foreign body). Atelectasis can mimic pneumonia, particularly when signs of volume loss, such as crowding of air bronchograms, fissural deviation, mediastinal shift, and diaphragmatic elevation, are absent. Flat, platelike opacities are characteristic of discoid atelectasis. Complete lung collapse, lobar collapse, or segmental collapse can also be seen. The characteristic LUS finding is a homogenous, well-demarcated hyperechoic lung consolidation. In comparison with pneumonia, no air bronchograms are usually visible. The size of the lung consolidation may vary during the breathing cycle due to ventilation. Atelectasis is categorized according to mechanism as obstructive, compressive, cicatricial, and adhesive.
1.4 Acute Aspiration
The term aspiration describes a variety of situations involving the intake of solid or liquid materials into the airways and lungs (Franquet et al. 2000). The clinical and radiologic manifestations are protean, varying from asymptomatic focal inflammatory reaction with few or no radiologic abnormalities to severe life-threatening disease (Table 5).
Table 5
Main syndromes of acute/recurrent pulmonary aspiration
Aspirated material | Clinical effects | Imaging findings |
|---|---|---|
Foreign bodies | Bronchial obstruction | Atelectasis |
Hyperinflation | ||
Abscess | ||
Water | (Near) drowning | Airspace opacity |
Gastric contents (Mendelson syndrome) | ||
Acute | Chemical pneumonitis, anaerobic infection, lung abscess | Airspace opacity |
Cavity | ||
Chronic/recurrent | Recurrent aspiration | Migratory opacities |
Bronchiolitis | Centrilobular nodules/air trapping | |
Bronchiectasis | ||
Acute exogenous | ||
Lipoid pneumonia (fire-eater hydrocarbon pneumonia) | Chemical pneumonia | Consolidations |
Pneumatoceles | ||
Masses | ||
Alcoholism is probably the most important predisposing factor for pulmonary liquid aspiration in adults, although other factors (e.g., general anesthesia, loss of consciousness, structural abnormalities of the pharynx and esophagus, neuromuscular disorders, deglutition abnormalities) may also contribute to aspiration.
Aspiration can result in airway obstruction, chemical pneumonitis, or infectious pneumonia, depending on the volume and type of aspirate. Patchy, ill-defined ground-glass, consolidative, and nodular opacities are the most frequently encountered radiographic manifestations of aspiration. Opacities typically appear rapidly and are most commonly located in the dependent regions of the lungs: the posterior segment of the upper lobes and the superior and posterior basal segments of the lower lobes (Franquet et al. 2000). Opacities may increase in conspicuity over the first 1–2 days in aspiration pneumonitis, but should resolve relatively rapidly thereafter. If opacities persist or increase over several days, aspiration pneumonia is likely present. Patchy, dependent ground-glass, and consolidative opacities are also seen on CT. “Tree-in-bud” opacities result from filling and inflammation of the distal airways (Rossi et al. 2005). When tree-in-bud opacities are present in a dependent distribution, they are highly suggestive of aspiration (Fig. 6).
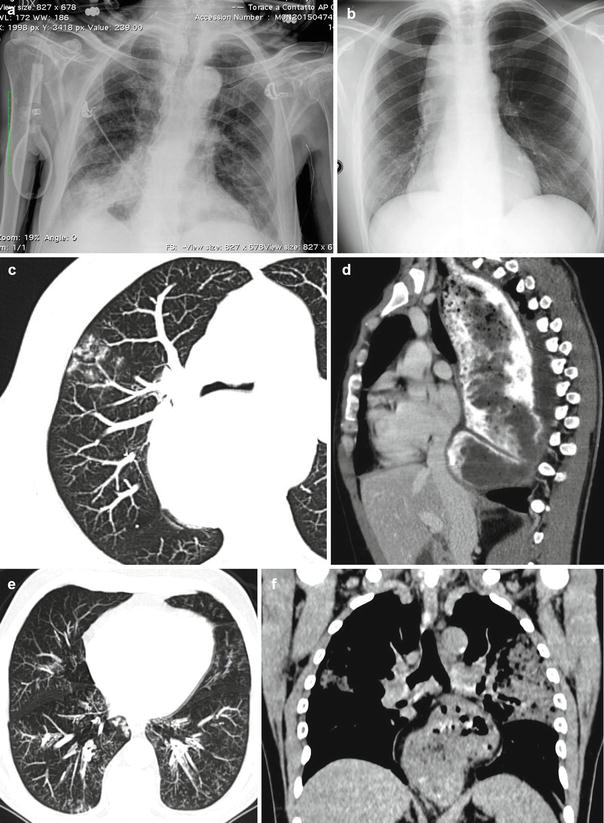

Fig. 6
Case 1. Acute aspiration pneumonia (Mendelson syndrome) in a 65-year-old man who had undergone surgery for intestinal obstruction. (a) Anteroposterior CXR view obtained 2 h after surgery demonstrates a focal consolidation in the right lower lobe, a finding that is consistent with aspiration pneumonia. Case 2. Aspiration bronchiolitis in a 57-year-old man with esophageal achalasia. (b) Frontal CXR view shows a right superior and middle paramediastinal mass. (c) Thin-section MDCT axial scan shows multiple diffuse centrilobular areas of increased attenuation with a characteristic tree-in-bud appearance (aspiration bronchiolitis); note that dilated esophagus displaces tracheal carina. (d) MDCT sagittal reconstruction shows typical findings of achalasia with contrast medium-filled esophagus, marked esophageal dilatation, and anterior bowing of the trachea. Case 3. Aspiration bronchiolitis and lung consolidations in a 46-year-old man with hiatal hernia. (e, f) Axial and coronal MDCT reconstruction shows centrilobular nodules, tree-in-bud pattern in lower lobes, consolidations in upper lobes, and large gastric hernia
1.4.1 Foreign Body Aspiration
Aspirated foreign bodies are by far the most common cause of intraluminal airway abnormalities in childhood, unusual in adults, and often overlooked as a cause of airway obstruction (Yadav et al. 2007). Up until age 15, both right and left main bronchi arise at about the same angle from the trachea so that objects may be aspirated into either side; afterward, the right main bronchus arises in a less acute, more straight path than the left (Kosucu et al. 2004). The most frequently aspirated foreign bodies are food (especially nuts), teeth, dental devices, and medical instruments, and 70 % will be nonopaque on conventional radiography (Fig. 7).
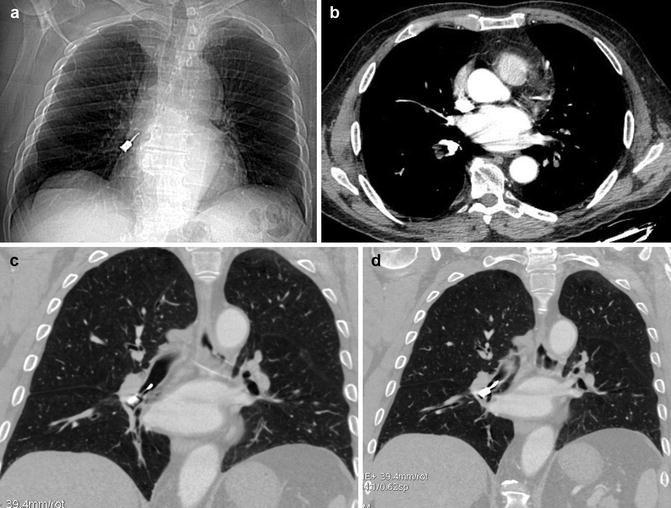

Fig. 7
Post-aspiration imaging in a 70-year-old alcoholic man. MDCT (a) scanogram and (b) axial and (c, d) coronal reconstruction images clearly show inhalation of a metallic foreign body (dental screwdriver), located in the right lower lobe bronchus
Some nuts, such as peanuts, have an oil that leads to inflammation and edema making them more difficult to expel. When a foreign body enters the lung parenchyma, prolonged irritation with intermittent infections may cause massive hemoptysis. At radiology, an aspirated foreign body may cause one lung or lobar/segmental overinflation or atelectasis (obstructive emphysema) with chronic volume loss in the affected lobe, mediastinal shift, pneumomediastinum, recurrent pneumonias, and bronchiectasis; it can occasionally mimic a congenital malformation or neoplasm (Fig. 8).
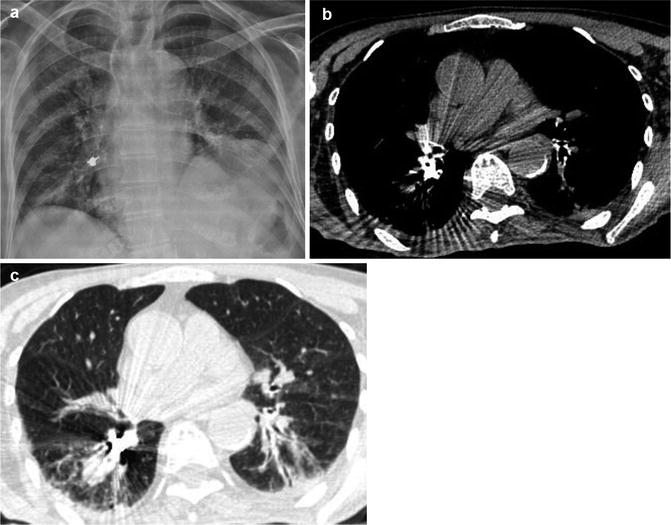

Fig. 8
Post-aspiration imaging in a 79-year-old woman. (a) CXR and MDCT with (b) mediastinal and (c) parenchymal windows show a foreign body inhalation, again located in the right lower lobe bronchus (earring)
If the patient is clinically able, an expiratory CXR may demonstrate air trapping on the affected side by lack of collapse of the lung and shift of the mediastinum away from the side with the foreign body. If the patient is a child or otherwise not able to cooperate for an expiratory study, a decubitus view of the chest, with the suspected side down, may show a lack of collapse of the air-trapped lung. CT may demonstrate the foreign body and is far more sensitive than CXR in demonstrating subtle low-attenuation intrabronchial material (Fig. 9) (Zissin et al. 2001).
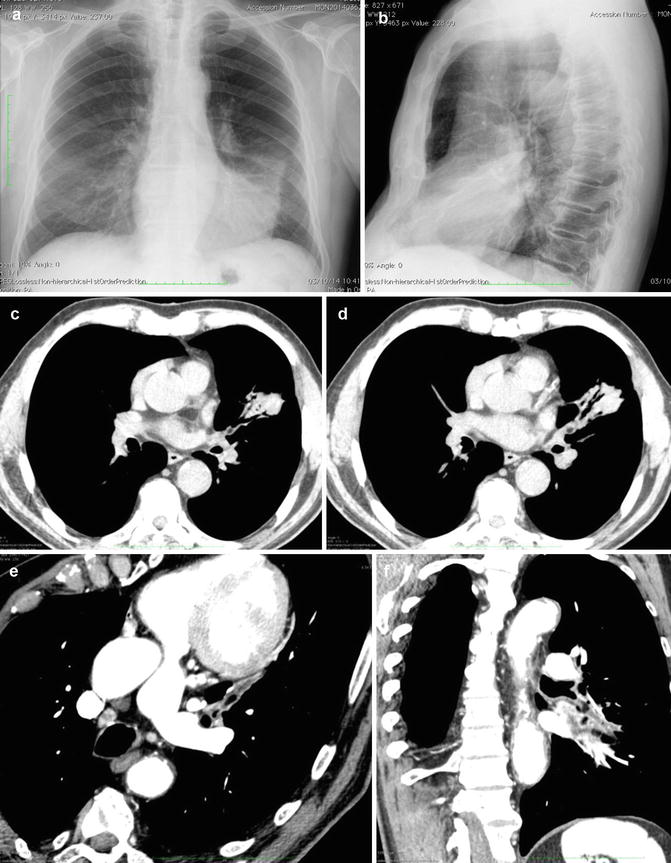

Fig. 9
Postobstructive atelectasis in a 46-year-old man who had inhaled vegetable lipid matter. (a) Posteroanterior and (b) latero-lateral CXR views show segmental lingular lung collapse, without evidence of foreign body. (c, d) Axial MDCT scans confirm lingular collapse and show possible endoluminal oval hypodensity. MDCT (e) oblique axial and (f) coronal MIP reconstructions show a lipid-density oval endobronchial mass. At bronchoscopy foreign body aspiration diagnosis was confirmed and a vegetable lipid matter was removed
1.4.2 Drowning or Near Nonfatal Drowning
Drowning is a process resulting in primary respiratory impairment from submersion in a liquid medium (Idris et al. 2003) and may be either a fatal or nonfatal event (Szpilman et al, 2012). Deaths because of drowning have estimated approximately 305,000 deaths in 2008 (WHO 2011). Young children are at particularly high risk of drowning, and males in general face a significantly greater risk than females. Drowning may be further classified as cold-water (<20 °C) or warm-water injury (>20 °C). The most important contributory factors to morbidity and mortality from drowning are hypoxemia and acidosis and the multiorgan effects of these processes. Central nervous system (CNS) damage may occur because of hypoxemia sustained during the drowning episode (primary injury) or may result from arrhythmias, ongoing pulmonary injury, reperfusion injury, or multiorgan dysfunction (secondary injury), particularly with prolonged tissue hypoxia. After initial breath holding, when the victim’s airway lies below the liquid’s surface, an involuntary period of laryngospasm is triggered by the presence of liquid in the oropharynx or larynx (stage 1, “dry drowning”). At this time, the victim is unable to breathe in air, causing oxygen depletion and carbon dioxide retention. In stage 2, the victim still usually presents with laryngospasm but may begin to swallow water into the stomach. As the oxygen tension in blood drops further, laryngospasm releases, and the victim gasps and hyperventilates, possibly aspirating variable amounts of liquid. This leads to further hypoxemia (stage 3). Ultimately, if the process of drowning is not interrupted, hypoxia progresses to the point of complete circulatory collapse and cardiac arrest (Tourigny and Hall 2012). Pulmonary infections may occur in a delayed fashion from aspirated material, some of which may result from rare or atypical pathogens such as fungi. Patients in cardiac arrest are more likely to display a nonshockable cardiac rhythm (i.e., asystole or pulseless electrical activity). Those who survive the initial resuscitation phase are at significant risk for developing serious delayed sequelae, such as ARDS, pancreatitis, disseminated intravascular coagulation, or rhabdomyolysis during their subsequent hospital stay. The acute aspiration of massive amounts of water produces a pulmonary edema that is radiographically indistinguishable from pulmonary edema from other causes (Hunter and Whitehouse 1974; Causey et al. 2000). The clinical significance of near drowning depends more on the volume of water aspirated than on whether the aspirate is freshwater or saltwater. Classic CXR findings in severe near drowning consist of alveolar edema with extensive “fluffy” areas of increased opacity that tend to coalesce throughout both lungs (Fig. 10). In mild near drowning, findings range from normal to confluent irregular peripheral areas of increased opacity in a subsegmental or segmental distribution with peripheral sparing. Pneumonia may be a complication of the aspiration of either fresh- or saltwater and, depending on the water source, may be caused by a variety of microorganisms including bacteria, fungi, and mycobacteria (Tourigny and Hall 2012).
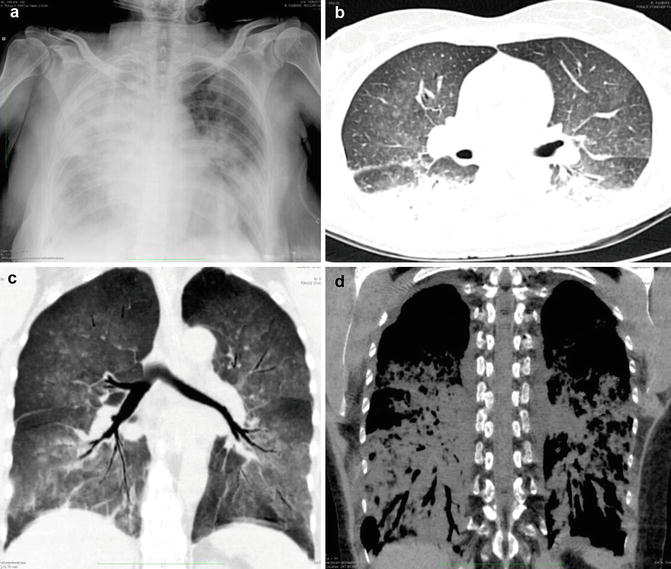

Fig. 10
Near drowning in a 67-year-old man who was admitted 3 h after nearly drowning in chlorinated water in an hotel pool. (a) CXR obtained at the time of admission reveals cardiac enlargement, diffuse confluent alveolar patterns of pulmonary edema, and peribronchial cuffing. Emergent MDCT (b) axial and (c, d) coronal reconstructions (parenchymal and mediastinal windows) show diffuse ground-glass and consolidative dependent abnormalities
1.4.3 Gastric Acid Aspiration (Mendelson Syndrome)
Vomiting with massive aspiration of gastric contents is a very frequent phenomenon; gastric acid with a pH <2.5 can cause pathologic reactions varying from mild bronchiolitis to hemorrhagic pulmonary edema. The posterior segments of the upper lobes and the superior segments of the lower lobes are the most frequently involved lung sites when the patient is recumbent. Acid liquid introduced into the airways is rapidly disseminated throughout the bronchial tree and lung parenchyma, producing a chemical pneumonitis within minutes. The magnitude of injury is directly related to the pH and volume of the aspirated material. The overall mortality rate associated with massive aspiration of gastric acid is approximately 30 % and is greater than 50 % in patients with initial shock or apnea, secondary pneumonia, or adult respiratory distress syndrome (Bynum and Pierce 1976). Classic CXR findings in acute gastric acid aspiration include bilateral perihilar, ill-defined, alveolar consolidations, multifocal patchy infiltrates, and segmental or lobar consolidation, which are usually localized to one or both lung bases (Fig. 6a).
1.4.4 Acute Exogenous Lipoid Pneumonia (Fire-Eater Pneumonia)
Exogenous lipoid pneumonia is a rare condition caused by inhalation or aspiration of plant, animal, or mineral fats and may take an acute or chronic form. Acute form is usually associated with accidental poisoning in children; in an adult population, it typically occurs in fire-eaters, who use oily substances in their shows (Betancourt et al. 2010). Clinically, acute lipoid pneumonia presents most often with cough, dyspnea, and fever. CXR pictures show consolidation, reticular and mixed alveolar-interstitial changes, as well as nodular lesions. Other rarer abnormalities, which may be noted on radiograms, are pneumatocele, pneumothorax, pneumomediastinum, and pleural effusion (Franquet et al. 2000). Most commonly, CT shows areas of a negative attenuation coefficient (between −150 and −30 HU) consolidation and ground-glass opacities as well as interstitial changes such as interlobular septal thickening and intralobular lines; fine, poorly demarcated centrilobular nodules and nodular lesions; pneumatocele; pneumothorax; pneumomediastinum; and pleural effusion (Brander et al. 1992; Laurent et al. 1999). Changes involve one or both lungs and are usually located in lower lobes or in the right middle lobe and may be multifocal or bilateral (Fig. 11).
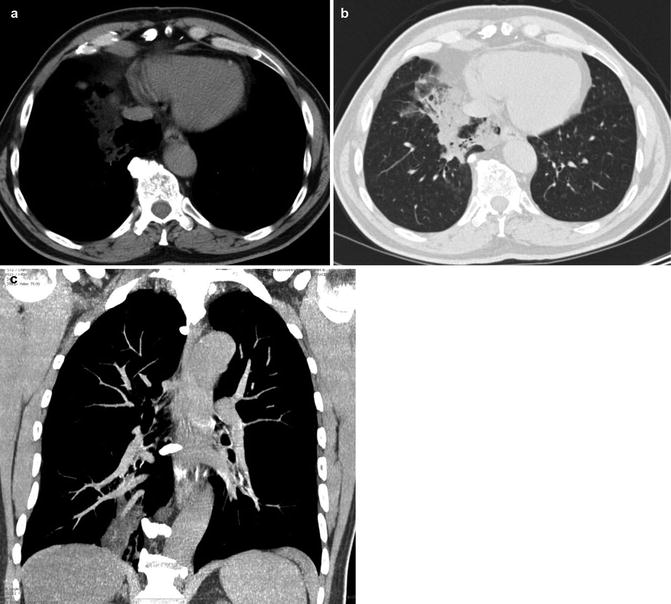

Fig. 11
Acute exogenous lipoid pneumonia. A 44-year-old actor performing in fire blowing shows who has aspirated liquid kindling for the grill 2 days before the onset of symptoms and was admitted acutely to the emergency department due to 38.8 °C fever, hemoptysis, pleuritic chest pain, and dyspnea. (a, b) Axial MDCT scans show a low-density consolidation in the middle lung lobe and scattered ground-glass opacities. (c) MDCT coronal MIP reconstruction better shows the low-density middle lobe consolidation. Region-of-interest measurements showed -60 to -80 Hounsfield units (HU), consistent with fat
1.5 Summary
Airway obstruction is an uncommon but potentially life-threatening condition that can be due to a number of malignant and benign processes; the diagnosis is based on the combination of characteristic findings on physical examination, as well as physiological, imaging, and endoscopic studies. Ultimately, the diagnosis is made by direct visualization of the tracheobronchial obstruction by bronchoscopy. Significant airway obstruction presenting with imminent suffocation requires immediate action to promptly and effectively reestablish and secure a patent airway and relieve the obstruction. Currently, the most comprehensive approach can be offered at centers with expertise in the management of acute complex airway disorders and availability of all endoscopic and surgical options.
2 Acute Exacerbation of Bronchiectasis
2.1 Introduction
The term bronchiectasis is derived from the Greek term bronkia (bronchial tubes), ek (out), and tasis (stretching), which together literally mean “the outstretching of the bronchi” (Feldman 2011). First described by Laennec in 1819, later detailed by Sir William Osler in the late 1800s, and further defined by Reid in the 1950s, this condition is generally defined as an abnormal and permanent focal or diffuse dilatation of the cartilage-containing airways (bronchi) caused by weakening or destruction of the muscular and elastic components of the bronchial walls (Hansell et al. 2008). Care must be taken to distinguish this large airway dilatation from dilatation of the small airways (bronchioles) that do not contain cartilage (Javidan-Nejad and Bhalla 2010). In contrast to established bronchiectasis occurring in adults, some children with bronchiectasis have been shown to have resolution or considerable improvement of the changes seen on CT scanning, suggesting the possibility that the condition may be reversible in some cases (Al Subie and Fitzgerald 2010).
Bronchiectasis results from the occurrence of one of the three main pathogenic mechanisms: bronchial wall injury, bronchial lumen obstruction, and traction from adjacent fibrosis (Shoemark et al. 2007). The latter two mechanisms are usually apparent on imaging and are suggested by an endobronchial filling defect or adjacent interstitial lung disease, while when it is met the first mechanism the radiologist is faced with a more difficult differential diagnosis (Javidan-Nejad and Bhalla 2009). The dominant feature of bronchiectasis is clearly the presence of airway inflammation, in association with bacterial infection and, in particular, nonclearing infection. An initial airway insult, such as an infection, often on the background of genetic susceptibility, compromised host clearance mechanisms and, in particular, the mucociliary escalator mechanism, which facilitated persistent bacterial colonization and infection. This damages the airway further, both directly and indirectly, as a consequence of the initiation of a secondary host inflammatory response. The three pathogenic elements in bronchiectasis—namely, airway infection, inflammation, and enzymatic activities—interact to result in progressive airway damage. Many patients eventually harbor Pseudomonas aeruginosa (PA) in their airway, which is associated with significant morbidity (Pappalettera et al. 2009).
The Reid morphological classification, derived from bronchographic and autopsy studies, specifies three patterns (Reid 1950):
- 1.
Cylindrical or tubular bronchiectasis in which there is relatively uniform dilatation of sections of the bronchial tree, involving diffuse mucosal edema, with resultant dilated bronchi but with straight, regular outlines and that end squarely and abruptly.
- 2.
Varicose bronchiectasis in which there are local constrictions superimposed on cylindrical bronchiectasis (bulbous or beaded appearance), with alternating areas of dilatation and constriction and, potentially, obstructive scarring that may subsequently result in postobstructive pneumonia and additional parenchymal damage.
- 3.
Saccular or cystic bronchiectasis in which there is progressive dilatation of the airways giving a balloon-like appearance and sometimes air–fluid levels; the number of bronchial divisions is greatly reduced; it is generally acknowledged that cystic bronchiectasis represents the most advanced disease (Fig. 12).
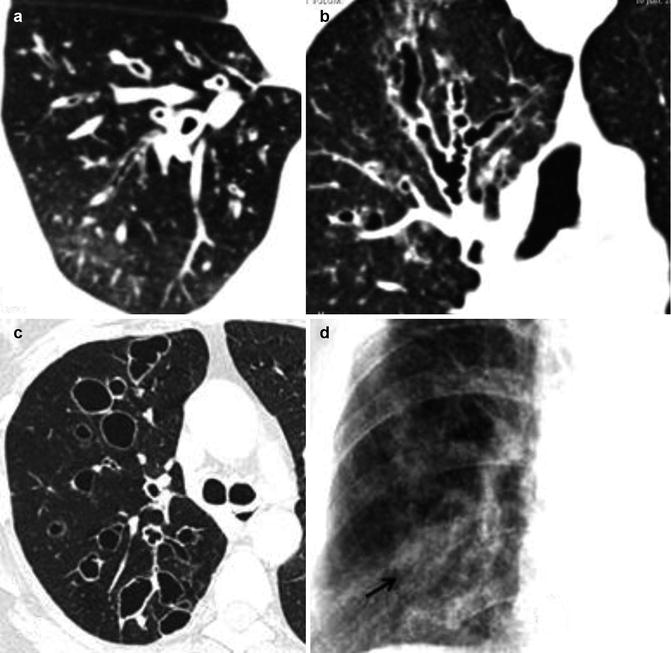
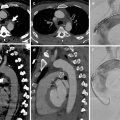
Fig. 12
The Reid morphological classification of bronchiectasis. (a) MDCT axial scan shows cylindrical or tubular bronchiectasis; (b) MDCT coronal reconstruction shows right upper lobe varicose bronchiectasis; (c) MDCT axial scan shows right upper lobe saccular or cystic bronchiectasis; (d) CXR close-up of the right lower lobe shows mucous plugging of bronchiectasis (arrow)
Oftentimes, more than one type of bronchiectasis can be seen in the same patient. As the airway dilatation increases, there may be progressive collapse and fibrosis of the distal lung parenchyma. Bronchiectasis can frequently occur in parallel with more common forms of chronic lung disease including COPD (up to 50 % of patients) and asthma (overlap syndromes) (Patel et al. 2004). There are many medical conditions that may lead to the development of bronchiectasis and these are detailed in Table 6.
Table 6
Causes of bronchiectasis
Causes of bronchiectasis | Imaging findings to provide clues to the diagnosis |
|---|---|
Idiopathic (48 %) | Often a lower lobe distribution |
Post-infective (25 %) | |
Severe pneumonia | |
Tuberculosis | |
NTM | Prominent nodularity in a tree-in-bud pattern or with cavitation |
Pertussis | Lower lobe predominance |
Measles | |
Impaired mucociliary clearance (9 %) | |
CF | Bilateral, proximal, parahilar, predominantly upper lobe disease |
Primary ciliary dyskinesia | 50 % associated with situs inversus, middle lobe/lingular predominance |
Young’s syndrome | Predominantly lower lobe disease |
Chronic obstructive pulmonary disease and smoking | |
Immunodeficiency (8 %) | |
Common variable immunodeficiency | |
Hypogammaglobulinemia | Disproportionate bronchial wall thickening |
Specific polysaccharide antibody deficiency | |
Secondary immunodeficiency, e.g., malignancy (chronic lymphocytic leukemia) | |
Human immunodeficiency virus infection | |
Lung and bone transplantation | |
Exaggerated immune response | |
Allergic bronchopulmonary aspergillosis | Upper lobes, central bronchiectasis, prominent mucus plugging |
Graft versus host disease | |
Inflammatory bowel disease (ulcerative colitis and Crohn’s disease) | |
c-ANCA-positive vasculitis | |
Rheumatoid arthritis (3.2–35 % of patients) | |
Congenital abnormalities of the bronchial wall | |
Mounier-Kuhn syndrome | (tracheobronchomegaly; enlarged tracheal diameter:>25 mm men, >23 mm women; trachea and bronchi of first to fourth order; central bronchiectasis) |
Williams-Campbell syndrome | (congenital absence of cartilage from lobar to first- to second-generation segmental airways) |
Marfan syndrome | |
Job syndrome (hyperimmunoglobulin E syndrome) | |
Inflammatory pneumonitis | |
Aspiration of gastric contents | |
Inhalational exposure (smoke, ammonia, chlorine) | |
Fibrosis (traction bronchiectasis) | |
Sarcoidosis, radiation fibrosis, end-stage HP | Predominantly upper lobes disease |
Idiopathic pulmonary fibrosis | Predominantly lower lobes disease |
Mechanical obstruction (postobstructive) | Unilateral focal bronchiectasis |
Foreign body | |
Tumor | |
Extrinsic compression (e.g., lymph node) | |
Miscellaneous conditions
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |