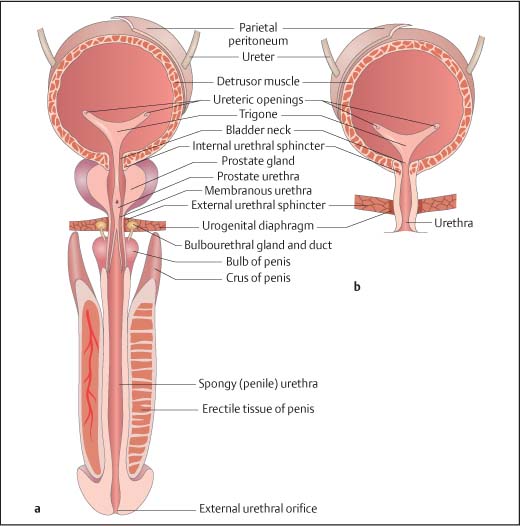
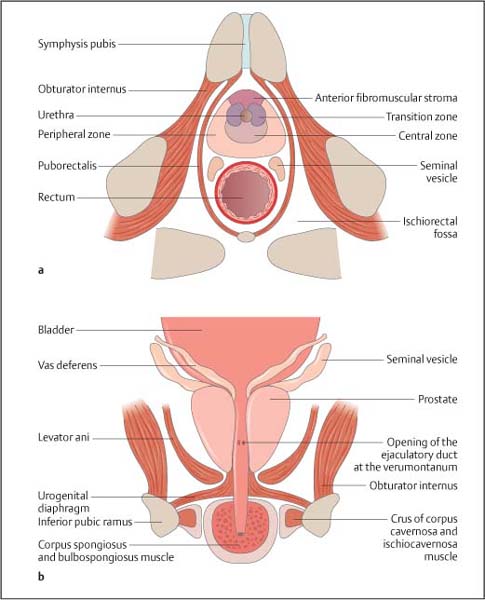
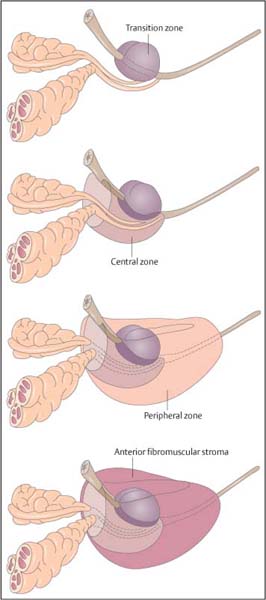
10 Diseases of the Bladder and Prostate Of all the radiological modalities, ultrasound is ideally suited for evaluation of the lower urinary tract, as it does not involve ionizing radiation, is reproducible and, apart from transrectal ultrasound (TRUS), is a noninvasive investigation. As a screening or firstfigline examination, ultrasound is unrivaled. The present chapter will detail the diagnostic value of lower urinary tract ultra-sound. The bladder develops from the urogenital sinus, but the base (or bladder trigone) is of mesonephric duct origin. The median umbilical ligament is a fibrous cord anterior to the bladder and develops from the urachus. The bladder lies centrally in the pelvis1 and, although extraperitoneal, the peritoneum reflects over its superior surface (Fig. 10.1). The bladder base or trigone lies inferiorly and is indistinguishable from the rest of the bladder, except it may be slightly raised, particularly in the male because of the prostate gland. The ureters enter the trigone approximately 2 cm either side of the midline and are identifiable as a slight “corrugation” as they traverse diagonally through the bladder wall. With full distension, the normal bladder neck may be seen as a slightly open funnel at the base of the bladder. These structures, the trigone, ureters, and bladder neck, are the only fixed landmarks seen on bladder ultrasound. The bladder wall is muscular and has three parts:The outer longitudinal muscle layer, the middle circular muscle, and an inner longitudinal layer. Fig. 10.1 Depiction of the gross anatomy of the male (a) and female (b) bladder Fig. 10.2 Anatomical depiction of the prostate gland. a Axial plane. b Coronal plane The urothelium (transitional cell epithelium) lines the inner muscle layer and is continuous with the urothelium of the ureters and urethra. The muscles around the bladder neck fuse to form the internal urethral sphincter, which encircles the upper prostatic urethra. In the female the external urethral sphincter lies just below the bladder neck and the uterus posteriorly; in the male the prostate gland lies between the bladder neck and the external urethral sphincter, with the seminal vesicles and rectum as the posterior structures (the rectovesical pouch lies in between). Embryologically, the prostate gland develops from outgrowths of the urogenital sinus and the embryological prostatic urethra. Postpuberty the gland has a volume of up to 25 mL. The prostate gland measures approximately 3.5 cm in length or height, 4.0 cm in width, and 2.5 cm anteriorly to posteriorly in depth, or the size and shape of a walnut. For unknown reasons, though presumably hormones play a role, the prostate gland increases in size and changes shape with age unless the man has been castrated before puberty, in which case the gland does not mature. The prostate gland is a retroperitoneal structure, lying anterior to the rectum and inferior to the bladder (Fig. 10.2). Between the gland and the rectum lies Denonvillier fascia; this is an obliterated peritoneal plane. The shape of the prostate gland conforms to the anatomical limitations of the deep pelvic boundaries and has the appearance of an inverted cone or pyramid. On the sides of the prostate gland lie the levator ani and obturator internus muscles. The superior margin, or the base of the prostate gland, lies immediately below the bladder. The most inferior part of the prostate gland, paradoxically the apex of the gland, lies just above the urogenital diaphragm, a fibrous supporting ring which also contains the urethra and the external urethral sphincter. The “prostate capsule” is not a true fibro-elastic capsule but a loose compression of the periprostatic fat and stroma, through which pass the neurovascular bundles and the ejaculatory ducts. Fig. 10.3 Anatomical depiction of the zonal anatomy of the prostate gland The deep pelvis is a restricted space and insufficient to accommodate significant prostate gland enlargement. Beyond a certain size, the prostate gland will preferentially enlarge superiorly, protruding into the bladder base, the so-called median lobe enlargement. In the past, the prostate gland was considered a lobar structure, with right and left lobes, and a midline median lobe. Lobar anatomy is no longer thought to be an accurate representation in the adult, and the gland is now split into glandular zones. The neurovascular bundles contain the branch arteries, veins, and nerves, and lie posterolaterally. Anatomically the gland may be divided into zones,2,3 which is more important than the gross anatomy, as zonal anatomy may be visualized on ultrasound and has a bearing on disease distribution and biopsy technique. Three glandular zones may be defined: The central, the peripheral, and the periurethral transition zones. There is a further anterior nonglandular area called the fibromuscular stroma (Fig. 10.3). The line between the peripheral and central/transition zones, referred to as the “surgical” capsule, separates the inner from the outer gland of the prostate and represents the line of dissection during certain surgical operations. The peripheral zones account for 75% of the prostate mass in young men, but with increasing age the transitional zone increases in size due to benign prostatic hyperplasia (BPH) whilst the central zone atrophies and the peripheral zone remains static. Therefore, for clinical purposes the important regions are the peripheral and transition zones. A separate terminology divides the gland into the peripheral and central glands (or the outer and inner glands), indicating that it is impossible to identify the central zone as a separate structure. The peripheral and central zones are terminologically incorporated into a single unit, namely the peripheral or outer gland. Summary points: • The prostate gland is split into three glandular zones: Central, peripheral, and transition zones • The prostate gland has the appearance of an inverted pyramid • The prostate gland has a normal volume of 25 mL • The prostate gland capsule is not a true fibroelastic capsule but is made up of periprostatic fat and stroma • The line between the peripheral and central/transitional zones (surgical capsule) separates inner from outer gland The prostatic urethra runs in the midline through the gland from the base of the bladder to the urogenital diaphragm (Figs. 10.2, 10.3). The prostatic urethra has no lumen at rest, but during micturition the cross-sectional area varies from round to triangular in the mid-portion. The triangulated portion is the verumontanum, and the ejaculatory ducts drain at this site. There is a variable amount of smooth muscle around the urethra and together with the urothelial margin, despite being collapsed; the urethra is visible on ultrasound. The external sphincter, and to a lesser extent the bladder neck, maintain continence. The seminal vesicles are paired sacs of variable shape and size that lie behind the bladder and above the prostate gland (Figs. 10.2, 10.3). A degree of asymmetry is common. Embryologically, the seminal vesicles represent outsourcings of the vasa deferens and when fully formed merge with the vas deferens to form the ejaculatory ducts, which enter the base of the prostate gland. The ejaculatory ducts run through the prostate in the central zone, in a close midline position. The normal nondilated ejaculatory ducts are difficult to separate from each other and commonly the two are referred to as the ejaculatory duct complex. The course of the ejaculatory ducts is anterior and they communicate with the posterior urethra at the verumontanum. Functionally the bladder and prostate gland are intimately linked, but ultrasound examination of the two requires separate approaches; transabdominal ultra-sound imaging for the bladder and TRUS imaging for the prostate gland. Although in certain situations the alternative ultrasound imaging approach may be informative for either structure. A full bladder is essential and a 3.5–5 MHz curved array probe is used. Only in a well-distended bladder can wall abnormalities be recognized, otherwise apparent focal wall masses or diverticula may be simulated by invaginations of the bladder wall. Supine position is adequate, but lateral scanning can help identify mobile calculi. A systematic method should be used: First the bladder is imaged in an axial plane and note made of any asymmetry of the wall. The normal bladder wall is smooth and thin. The normal wall thickness is quoted as 3–5 mm and measures < 3 mm when well-distended.4 Next, the bladder is imaged in the longitudinal plane. Asymmetry of the bladder wall is again assessed. In the midline, the bladder neck is seen as a short funnel. The lower ureters should be particularly scrutinized and both axial and longitudinal views are useful. Again, asymmetry is important and calculi lodged in the ureters may be suspected by a prominence of the intramural portion of the ureters. Transrectal imaging may also be used to visualize the intramural ureter and ureteric orifice.
Introduction
Anatomy of the Bladder and Prostate
The Urinary Bladder
The Prostate
Gross Anatomy of the Prostate
Zonal Anatomy of the Prostate
Anatomy of the Prostatic Urethra
Anatomy of the Seminal Vesicles and Ejaculatory Ducts
Ultrasound of the Bladder and Prostate Gland
Ultrasound of the Bladder
Technique (Table 10.1.)
Preparation: | Full bladder; at least 200 mL, preferably > 400 mL |
Position: | Supine (or oblique/decubitus) |
Transducer: | 3.5-5 MHz curved array transducer. 7.5-10 MHz may be useful for the anterior wall (± harmonic/pulse-inversion imaging) |
Metliod: | Image systematically in both axial and longitudinal planes |
Images: | Axial and longitudinal positions |
Assess: | |
1. Bladder wall | |
a) Thickness: Normally <3 mm (range: 3-5 mm) when well-distended | |
b) Trabeculation | |
c) Focal masses: Location, solid or cystic, continuation above bladder wall, acoustic shadowing, mobile (use decubitus imaging) | |
2. Diverticula | |
Location, size or diverticulum ana necK, calculus, or mass witnin | |
3. Bladder base | |
a) Ureteric orifices and intramural ureter, calculi, ureterocele. Jets | |
b) Bladder neck | |
c) Base elevation; prostate enlargement | |
4. Scrutinize the blind areas (± harmonic/pulse-inversion imaging) | |
a) Assess anterior wall using a 5-7.5 MHz transducer | |
b) Lateral walls; anaulate the transducer | |
c) Bladder base: Transrectal transducer if suspicion persists | |
5. Bladder volume = height × width × depth × 0.52 | |
6. Urinary flowmetry and postvoid residue (if appropriate) | |
Often bladder ultrasound is combined with measurement of urinary flow rates and bladder emptying as the ultrasound cystodynamogram. Such “functional” ultrasound is useful in the assessment of bladder out-flow obstruction.
Ultrasound Appearances of the Normal Bladder
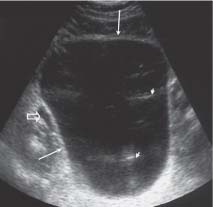
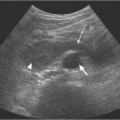
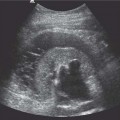
The normal bladder wall is smooth and any focal variation in wall thickness should be scrutinized for mucosal-based masses or diverticula (Fig. 10.4). Occasionally the different layers of the bladder wall can be differentiated but not sufficiently well for accurate analysis (e. g., for staging of bladder cancer). Well-distended, the ovoid shape of the bladder in the longitudinal plane and quadrangle in the axial plane is apparent. The bladder base is smooth in outline and the intramural ureters seen as linear “corrugations” along the bladder base. In the absence of an enlarged prostate gland the bladder base should not be elevated. Urine appears completely echo-free, but occasionally the more concentrated urine may layer posteriorly or be seen as a high-reflective “jet” as it ejects from the ureter.5
Fig. 10.4 Ultrasound image in the axial plane of a normal, well-distended bladder, demonstrating the thin bladder wall (long arrows) and the iliac vessels (open arrow). Reverberation artifact is present within the lumen of the bladder (short arrows)
Preparation: | None specific |
Position: | Left lateral position |
Transducer: | 5-10 MHz axial/longitudinal or end-firing transducer |
Metliod: | Image gland in both planes, base to apex and side to side |
Images: | Recorded in a) Transverse/axial planes; base, mid, and apex b) Longitudinal/sagittal planes; midline, left, and right parasagittal |
Assess: | |
1. Measure prostate volume (height × width × length × 0.52) | |
2. Note reflectivity, nodules, and gland symmetry | |
3. Evaluate the prostate capsule | |
4. Scrutinize blind areas in both planes: Posterolateral margins (also sometimes called anterior horns), base, apex, and far anterior gland (decrease frequency to 5-7.5 MHz) | |
5. Neurovascular bundle symmetry | |
6. Seminal vesicle and eiaculatory duct assessment | |
7. Color Doppler imaging (transducer movement, calcification, and biopsy will result in artifact signals) | |
N.B.: Prostate gland volume is more accurately measured by planimetry. The formula above becomes increasingly inaccurate with glands > 80 ml.
The echo-free urine makes bladder-wall analysis straightforward; but marked reverberation artifact may be seen and the anterior wall, lateral wall, and base of the bladder may be difficult to visualize. Suspected abnormalities of the anterior wall should be evaluated with a higher frequency probe (7.5– 10 MHz); the base may be better evaluated by transrectal imaging. Tissue-harmonic imaging helps to better define the bladder wall and reduce reverberation artifacts.
On color Doppler ultrasound the normal bladder wall does not demonstrate color signal, but urine may be seen ejecting from the ureteric orifices as “color jets.”6 Differences in the specific gravity between the ejecting urine and the bladder urine may account for the visualization of these jets on B-mode imaging alone.7 Visualization of jets is improved by hydration (500 mL of water before imaging or use of intravenous hydration or diuretic agents). Normal jets are well-defined, generally symmetrical cones of color turbulence, directed anteromedially.
Transrectal Ultrasound of the Prostate Gland
Technique (Table 10.2.)
Although the prostate gland may be visualized on transabdominal imaging, the views are often inadequate for diagnosis; dimensions may be measured, albeit inaccurately. In contrast, the prostate may be visualized in great detail using a transrectal probe. A variety of transducer designs are now available, each requiring a different method for imaging and transducer movement. Most radiology departments prefer the more versatile end-firing transducer which requires a fanning or rocking motion, unlike the simple advancement or rotation movements necessary for true axial/longitudinal transducers. These modern transducers image at a frequency of 5–10 MHz, but are often also capable of multifrequency imaging and prostate biopsy. A 7.5–10 MHz transducer is adequate for most prostate glands, but large prostate glands require 5 MHz to inspect the anterior structures or the bladder neck.
The left lateral patient position is best for imaging the prostate via the transrectal route. A lubricated, condom-covered transducer is inserted into the anus and directed slightly posterior to follow the natural contour of the lower rectum. Deep inspiration through an open mouth helps to relax the anal sphincter. The transducer should be advanced about 10 cm beyond the anal margin and the prostate gland examined in a systematic manner (Table 10.2). Magnification should be adjusted until the entire prostate gland is visualized on the ultrasound monitor. The prostate gland should be examined in the axial plane from the base to the apex. The reflectivity of the glandular zones should be compared between the two sides and note made of any asymmetry. The prostate gland should then be examined in the longitudinal plane and asymmetry again noted. Unlike the bladder, color flow imaging may be of diagnostic value and in the axial plane any focal color Doppler signal asymmetry noted. Following full examination, prostate gland biopsies may be obtained.
Ultrasound Appearances of the Normal Prostate Gland
In the young man, the prostate gland may be homogenous and the zones difficult to differentiate (Fig. 10.5), but normally the peripheral zone is hyperreflective relative to the central and transition zones. This echo-differentiation enhances further with glandular enlargement as the peripheral zone is compressed. Usually the central and transition zones cannot be separated from each other and the anterior fibromuscular stroma is not readily defined. Although there is no histological prostate capsule, the distinction between the gland and surrounding fat is so distinct that a “sono-graphic” capsule may be seen. The levator ani muscles are seen as linear echogenic boundaries of the prostatic bed. The urethra is seen as a line of mixed echogenicity, even though it has no lumen at “rest.” With muscular hypertrophy the urethra and bladder neck become increasingly of low reflectivity, with acoustic shadowing, a common finding with prostate gland enlargement. Just beyond the apex, the periurethral tissues are hypoechoic due to the external urethral sphincter. High-reflective, nonshadowing foci may be seen within the ducts of the inner gland, so-called corpora amylacea, representing mucoproteins of no clinical significance. Scattered areas of calcification are also sufficiently common to be considered a “normal” finding. More focal calcification may represent prostatitis or calculi within the ejaculatory ducts or urethra; only very rarely is prostate carcinoma calcified.
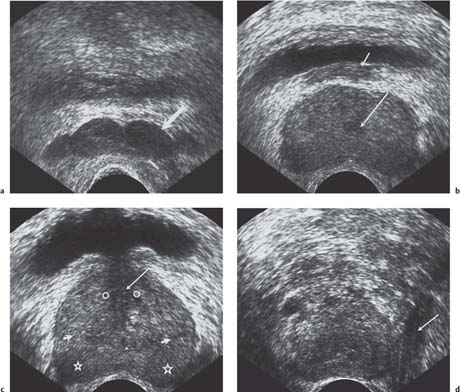
Fig. 10.5 Ultrasound appearances, in the transverse plane at several levels, of the normal prostate gland and seminal vesicles. a The seminal vesicles (arrow) lie posterior to the bladder base. b The base of the prostate gland, demonstrating the ejaculatory ducts (long arrow) and the seminal vesicles (small arrow). c The midportion of the prostate gland, demonstrating the urethra with surrounding low-reflectivity representing hypertrophy of the urethral muscles (long arrow). The peripheral zone (star), central zone (short arrow), and the transitional zone (open circle) are all demonstrated. d The apex of the prostate gland, demonstrating the apical portion (short arrow) and the levator ani muscle (long arrow).
Fig. 10.5e, f see next page
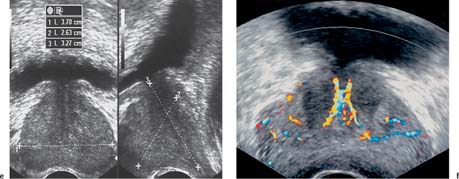
Fig. 10.5e, f e The dimensions of the prostate gland are measured in three directions to give an estimation of the gland volume (see Table 10.2). f Normal prostate gland vascularity with symmetrical flow around the urethra, periphery, and the intersection of peripheral and central zones is demonstrated on a color Doppler image
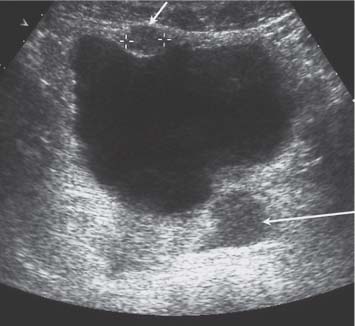
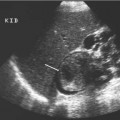
Fig. 10.6 A longitudinal view of the bladder with a solid urachal remnant (short arrow) at the upper aspect and the normal prostate gland at the lower aspect of the bladder (long arrow)
On color Doppler ultrasound the normal gland has marked vascular signals from the neurovascular bundles, the anterior venous plexus, the periurethral tissues, and vessels traversing the inner gland. Importantly, like the overall gland reflectivity, parenchymal vascularity should be symmetrical.8
Summary points:
• The peripheral zone accounts for 75% of the volume of the prostate gland in young men
• The transition zone increases in benign prostatic hypertrophy, the central zone atrophies, and the peripheral zone remains stable
• The clinically important zones are the transition and peripheral zones
• The peripheral zone of the prostate gland is high-reflective compared to the central/transition zone
• There is marked but symmetrical vascularity of the prostate as seen on color Doppler ultrasound
Ultrasound of the Abnormal Bladder (Table 10.3.)
Congenital Anomalies
Bladder agenesis is rare but duplication is slightly more common. Bladder duplication often presents with an incomplete septum; a complete septum dividing the bladder is very unusual.9 Congenital bladder anomalies, for example bladder extrophy, are usually identified in early childhood. However, urachal abnormalities may go undetected in childhood and present in the adult. A urachus may fail to involute and be seen as a patent tube (50%), a cyst in the midline (30%), a midline urachal sinus (15%), an anterior bladder diverticulum, or an area of thickening.10,11 There is an increased incidence of adenocarcinoma or calculus formation within a urachal remnant, and when a urachus is encountered, a soft-tissue mass or calculus should be carefully sought (using a 7.5–10 MHz linear array transducer; Fig. 10.6). Congenital diverticula are rare, but the Hutch diverticulum is characteristically wide-mouthed, small, and near the ureteric orifices.
Summary points:
• There is a higher incidence of adenocarcinoma or calculus formation in an urachal remnant
• A persistent urachus may take the form of a tube, a cyst, a sinus, or anterior bladder-wall thickening/diverticulum
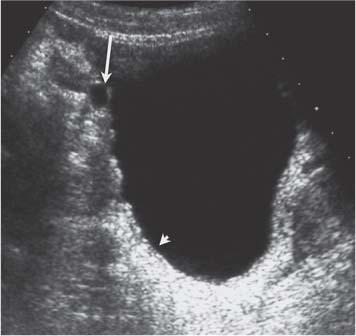
Acquired Diverticula
These are pulsion diverticula, secondary to the high voiding pressures of bladder outflow obstruction (Fig. 10.7). Acquired diverticula are usually associated with generalized bladder-wall thickening and trabeculation, have a narrow neck, and may be quite large. Any diverticula should be carefully examined for calculi and primary bladder tumors. When present, bladder diver-ticula, especially if there is a narrow neck, should be reported to the cystoscopy operator to allow for careful examination of mucosal-based lesions within the diver-ticula. A large diverticulum may act as a reservoir, resulting in incomplete emptying or “pis en deux,” recognized by comparing the prevoid and postvoid dimensions of the diverticulum.12 Such dynamic information is helpful when a patient is being considered for resection of a diverticulum, for example in those with recurrent infections or calculi.
Fig. 10.7 Thickened irregular bladder wall (short wall) with a small diverticulum (long arrow) in a patient with prostate gland enlargement, outflow obstruction, and high-pressure voiding
1. Diffuse bladder-wall thickening |
a) Bladder outflow obstruction: Trabeculated, small or large volume, diverticula, ureteric dilatation, enlarged prostate, bladder neck may be open or closed |
b) Neurogenic bladder: Trabeculated wall, small volume, ureteric dilatation (sometimes bladder is thin-walled and large)15 |
c) Tuberculosis: Small volume, ureteric strictures, rarely intravesical tuberculoma18,19 |
d) Schistosomiasis: Chronic, due to ova in the submucosa, focal masses may be seen, calcification20,21 |
e) Acute cystitis: Due to E. coli, fungal infection, emphysematous (air in bladder wall);17 there may be lobulated masses |
f) Hemorrhagic cystitis: May be drug-induced with lobulated masses |
g) Chronic cystitis: Interstitial cystitis, small, thick-walled22 |
2. Focal bladder-wall thickening |
a) TCC: Usually polypoid or “frondlike,” rarely plaque ± calcification |
b) Squamous cell carcinoma: Associated with Schistosomiasis,21 leukoplakia, chronic calculus disease, long-term catherization, or squamous metaplasia |
c) Adenocarcinoma: Arises from urachal remnant, anterior wall |
d) Leiomyoma, pheochromocytoma, lymphoma: No typical features |
e) Infections: Tuberculosis, acute schistosomiasis, malacoplakia,22 cystitis, cystica glandularis23 |
f) Amyloidosis |
g) Endometriosis24 |
h) Hemangioma: Serpiginous, cystic areas27 |
3. Intraluminal abnormalities |
a) Calculus: Shadow-casting, “twinkle” artifact |
b) Thrombus: High-reflective rim or layers of different reflectivity |
c) Fungus balls |
d) Indwelling catheter or foreign body |
4. Abnormalities of bladder outline |
a) Diverticula: May contain calculus, tumor |
b) Dilated ureters |
c) Ureterocele (may contain calculus) |
d) Urachal remnant cyst: Anterior wall |
e) Reimplanted ureters: Focal thickening or reflective nodule |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree