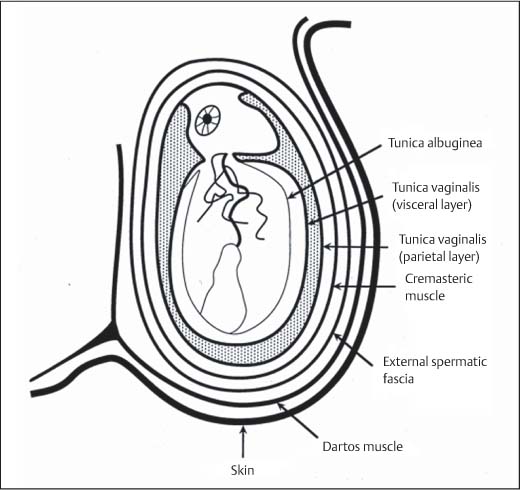
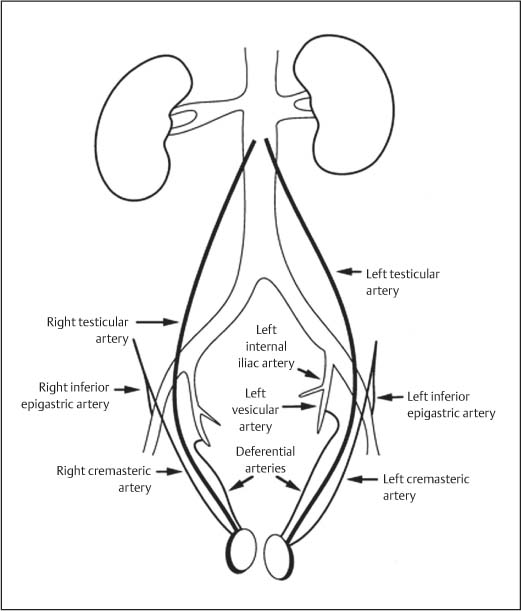
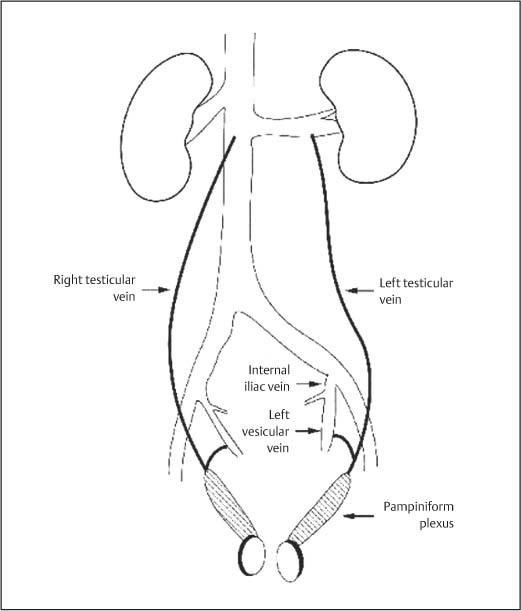
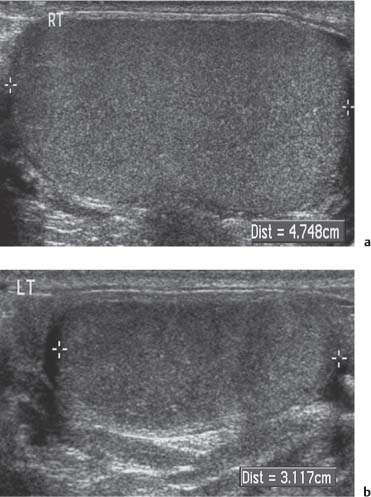
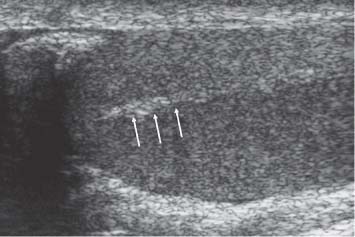
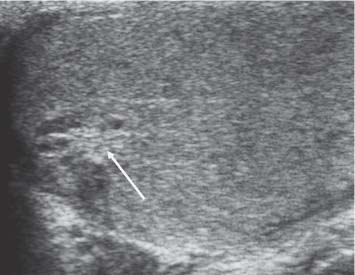
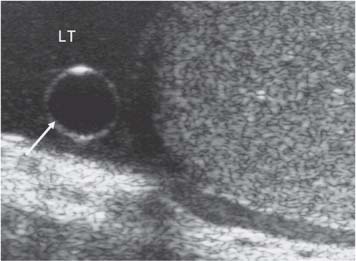
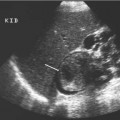
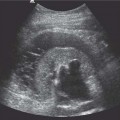
11 Diseases of the Testis and Epididymis The superficial nature of the scrotal sac and contents lends itself to detailed and precise imaging with ultra-sound. As a consequence, ultrasound is firmly established as the first-line and often only imaging modality employed in the assessment of scrotal abnormalities. Recent technical advances in transducer design and image processing have further improved ultrasound diagnosis of diseases of the scrotal contents. This chapter will deal with aspects related to the testis and epididymis, detailing both normal ultrasound features and those features related to disease processes. During the seventh month of fetal development, as a result of the rapid growth of the fetal body and failure of growth of the gubernaculum testis, the testes descend into the scrotal sac. A dense layer of fibrous connective tissue called the tunica albuginea forms a capsule that covers the testis. The testis is then further covered by a reflected fold of the processes vaginalis that becomes the visceral layer of the tunica vaginalis, with the remainder of the peritoneal sac forming the parietal layer of the tunica vaginalis. The visceral layer of the tunica vaginalis covers the testes and the epididymis, whereas the parietal reflection covers the anterior and lateral parts of the testes and the epididymis, leaving a “bare area” to which the mesentery of the testis is attached. A reflection of the tunica albuginea forms the mediastinum testis, within which the rete testis forms (Fig. 11.1).1 The arterial supply to the scrotal sac and contents arise from three sources: The testicular artery (arising from the aorta and supplying the testis), the cremasteric artery (a branch of the inferior epigastric artery, supplying the scrotal sac and the coverings of the spermatic cord), and the artery to the ductus deferens (arising from the superior vesicle artery) (Fig. 11.2). Veins exit the testes at the mediastinum; join the veins draining the epididymis to form the pampiniform plexus at the superior aspect of the testes. The cremasteric plexus (mainly draining extratesticular blood) lies posterior to the pampiniform plexus. The right testicular vein drains directly into the inferior vena cava (IVC) below the level of the right renal vein, whereas the left testicular vein drains into the left renal vein (Fig. 11.3). These three arteries and the veins are loosely held together by connective tissue along with nerves, lymph vessels, and the ductus deferens in the spermatic cord.2Although there are anastomoses between these arteries, these are not sufficient to prevent testicular ischemia when the testicular artery is compromised. Fig. 11.1 Anatomical layers surrounding the normal testis. The shaded area between the two layers of the tunica vaginalis is the area of fluid accumulation giving rise to a hydrocele (shaded area, potential space for fluid accumulation). (Source: Sidhu PS. Clinical and imaging features of testicular torsion: role of ultra-sound. Clin Radiol 1999;54:134–143) Fig. 11.2 Normal arterial anatomy of the testis. (Source: Sidhu PS. Clinical and imaging features of testicular torsion: role of ultrasound. Clin Radiol 1999;54:134–143) • The tunica albuginea covers the testis; a reflection forms the mediastinum testis • Two layers of tunica vaginalis (visceral and parietal) leave a small bare area • Three arteries supply the scrotum: The testicular artery, the cremasteric artery, and the artery to the ductus deferens • Pampiniform plexus drains into the testicular vein Fig. 11.3 Normal venous drainage of the testis. (Source: Sidhu PS. Clinical and imaging features of testicular torsion: role of ultrasound. Clin Radiol 1999;54:134–143) Examination should always take place in a private setting, with the person undertaking the examination using a gloved hand for the examination. A chaperone should be present. The ultrasound gel should be warm, and copious amounts applied. The scrotal sac may be held in a stable position by placing paper towels beneath the sac, and the penis should be held against the anterior abdominal wall, by the patient, and preferably covered with paper towels. By crossing the legs the patient is able to provide for more a stable position for the examination. A high-frequency linear array probe (7–12 MHz) should be used, with sensitive color and spectral Doppler capabilities. Typically the probe length should be such as to allow longitudinal length measurements of the testis. Initially both testes are examined in the trans-verse plane, in order to produce the “spectacle” view to allow comparison of testicular parenchyma features, which is important if a unilateral global testicular problem is suspected (Fig. 11.4). The examination of the entire scrotal sac should include both the transverse and longitudinal planes, documenting any abnormalities present. Testicular volume may be calculated and color Doppler ultrasound will confirm vascular supply. Summary points: • In US examination of the testes, a high-frequency linear array transducer is used • Sensitive color Doppler facility • “Spectacle” transverse view through both testes is essential The testes are homogenous and of medium-level reflectivity (Fig. 11.5). At birth the testis measures approximately 1.5 cm in length and 1.0 cm in width, and before age 12 the testicular volume is 1–2 mL. In the adult, testicular length may be up to 5 cm. Volume measurement is calculated using the formula length × width × height × 0.51. A total volume (both testis) of > 30 mL is indicative of normal function.3 A testicular volume > 2 mL allows reliable appreciation of intratesticular color Doppler flow.4 The mediastinum testis is seen as a highly reflective linear structure at the posterior–superior aspect of the testicle, draining the seminiferous tubules of the testes into the rete testis (Fig. 11.6). The rete testis is a low-reflective area at the hilum of the testis with fingerlike projections into the parenchyma (Fig. 11.7).5 Apart from these projections, the parenchyma of the testis should remain of homogenous reflectivity. The appendix testis (a vestigial remnant of the müllerian duct) is present in the majority of patients, most commonly at the superior testicular pole or in the groove between the testis and the head of the epididymis medially.6 There is marked variation in the size and appearance of an appendix testis; it is usually oval, although a stalklike cystic structure is occasionally seen (Fig. 11.8). Fig. 11.4 Transverse view through both the testes. The “spectacle view” allows comparison of the reflectivity of the two testes, of particular importance in infiltrative lymphoma and leukemia. In this example there is atrophy and altered reflectivity of the left testis following surgery for cryptorchidism Fig. 11.5 a The right testis from the same patient as in Fig. 11.4. It measures 4.7 cm in length and demonstrates normal, smooth reflectivity. b The left testis in the same patient is smaller, measuring 3.1 cm, and demonstrates a less uniform reflectivity Fig. 11.6 The mediastinum testis is seen as a highly reflective linear structure at the posterior–superior aspect of the testicle (arrows) draining the seminiferous tubules of the testes into the rete testis Fig. 11.7 The rete testis is a low-reflective area at the hilum of the testis with fingerlike projections into the parenchyma (arrow) The epididymis is 6–7 cm in length. The head (globus major) is a pyramid-shaped structure lying superior to the upper pole of the testis. The body courses along the posterolateral aspect of the testicle. The tail (globus minor) is slightly thicker than the body and can be seen as a curved structure at the inferior aspect of the testicle where it becomes the proximal portion of the ductus deferens. The body and tail are of similar or slightly lower reflectivity when compared with the testis, whilst the head is of slightly higher reflectivity (Fig. 11.9). Color Doppler signal may be identified in the normal epididymis.7 The appendix epididymis is not as frequently seen as the appendix testis.8 It is part of the mesonephric (wolffian duct), and projects from the epididymis from different sites, most commonly the head. It usually has a stalklike appearance. The globus major measures 10–12 mm in diameter, the body less than 4 mm (average: 1–2 mm) in diameter.9 Summary points: • The normal testis is of medium-level, smooth reflectivity • The mediastinum testis is a linear, high-reflective structure • The epididymal head is a pyramidal structure; the epididymal body is of lower reflectivity • The testicular and epididymal appendix are present at the superior aspect An intratesticular artery traverses the testis in a centrifugal direction in a reported 52% of patients, unilaterally in half.10 The artery is readily identified with color Doppler ultrasound, and returns a low-resistance spectral Doppler waveform (Fig. 11.10). Fig. 11.8 There is marked variation in the size and appearance of an appendix testis. It is usually oval, although a stalklike cystic structure is occasionally seen (arrow) Fig. 11.9 Changes in reflectivity of the epididymis. The low reflectivity of the body (long arrow) alters in the head of the epididymis (short arrow) to a higher reflectivity Fig. 11.10 Color and spectral Doppler ultrasound of transmediastinal vessels demonstrate the normal low-resistance pattern of the testicular artery and a normal venous waveform pattern Fig. 11.11 a There is a well-demarcated, low-reflective appearance generated through the testis (arrows) which does not appear to be related to a pathological cause. b Following the use of color Doppler ultrasound, the cause of this appearance is a prominent transmediastinal artery and vein Fig. 11.12 A florid example of a rete testis, with a number of cysts of varying size present adjacent to the mediastinum of the testis The term “two-tone” describes the appearance of an artifact within the testis where an intratesticular artery produces acoustic shadowing, resulting in a discreet uniform area of decreased reflectivity posterior to the artery (Fig. 11.11). This artifact is caused by refractive shadowing at both edges of the intratesticular artery.11 The reflectivity of the remainder of the testis is normal. The use of color Doppler ultrasound readily confirms the presence of the intratesticular artery as being the source of the artifact. The rete testis is located in the mediastinum testis (Fig. 11.12). Microscopically the rete testis is composed of three parts: The septal (interlobular) portion containing the tubuli recti, the tunical (mediastinal) portion consisting of a network of channels, and the extra-testicular rete, comprising the irregular-shaped lacunar spaces which connect to the efferent ducts.12 Seminiferous tubules contained within 250 lobules join the tubuli recti, and the efferent ducts drain out into the epididymis. On ultrasound, the rete testis has a spectrum of appearances ranging from a faintly visible ill-defined area of decreased reflectivity (18% of patients) at the testicular hilum to a coarse tubular appearance with fingerlike projections into the parenchyma.5 A remnant of the paramesonephric and mesonephric ducts may remain to form the appendix testis (hydatid of Morgagni) and appendix epididymis, respectively. Three further appendages, not seen on ultrasound, have been identified microscopically: The paradidymis (appendix of the cord or organ of Giraldés) arising from the spermatic cord and the superior and inferior vas aberrans of Haler.13 The appendix testis is found at the upper pole of the testis in the groove between the testis and the laterally situated head of the epididymis. The appendix testis can measure between 1 and 7 mm in length, and may be present in up to 92% of patients, bilaterally in 69%.14 The appendix testis is usually of similar reflectivity to the head of the epididymis, best seen in the presence of a hydrocele, with a variable morphology, most commonly being oval-shaped and sessile, but it may appear “stalklike” and pedunculated, cystic, or even calcified.6 The “stalklike” and cystic appendices are associated with an increased likelihood of appendiceal torsion, recognized as a cause of acute scrotal pain.15 The epididymal appendix is less frequently seen on ultrasound (6%), has a length of between 3 and 8 mm, is more frequently stalked, and, like the testicular appendix, may undergo torsion, although less commonly so.14 On occasion both an epididymal and testicular appendage may be seen in the same patient (Fig. 11.13). Fig. 11.13 Two areas (arrows) arising from the upper aspect of the testis. Both are isoreflective to the epididymal head, surrounded by a small hydrocele. The image demonstrates both the appendix testis and the appendix epididymis; differentiation is not possible on this image Fig. 11.14 A normal testis (long arrow) with a normal epididymal head (short arrows) and a further mass. It is isoreflective to the normal testis (arrowhead) lying in a superior position, representing an extra testicle: Polyorchidism Polyorchidism (more than two testes) is a rare condition and most commonly involves a bifid or duplicated testis with a single epididymis, with a uniform surrounding tunica albuginea (Fig. 11.14).16,17 Less common is a complete duplication of the testis and epididymis. On ultrasound, the extra testis demonstrates reflectivity identical to the normal testis.18 Testicular carcinoma represents 1% of all neoplasm in men and is the most common malignancy in the 15–34-year-old age group.19 A second peak prevalence occurs in the 71–90-year-old age group, with metastasis and lymphoma most common. There has been an unexplained increase in the prevalence of testicular carcinoma over the last 70 years, and testicular carcinoma is predominantly a cancer of white males. The most common presenting symptom is a painless scrotal mass; only 10% of patients present with pain. A smaller number of patients present with metastases or rarely with endocrine abnormalities such as gynecomastia. Survival rates for testicular carcinoma approach 95%.19 There are well-documented risk factors for the development of testicular carcinoma, namely previous testicular tumor, family history, cryptorchidism, infertility, and intersex syndromes. Testicular tumors may be divided into germ cell and nongerm cell tumors; 95% of testicular tumors are germ cell tumors which arise from spermatogenic cells. Nongerm cell tumors derive from sex cords (Sertoli cells) and stroma (Leydig cells); these tumors are malignant in 10% of cases. Lymphoma, leukemia, and metastases may manifest as testicular tumors (Table 11.1).
Introduction
Normal Anatomy
Ultrasound Examination Technique
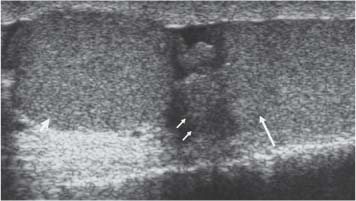
Normal Ultrasound Appearances of the Testis and Epididymis
Intratesticular Abnormalities
Normal Variants
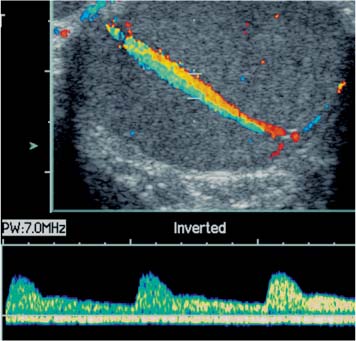
Transmediastinal Artery
Two-Tone Testis
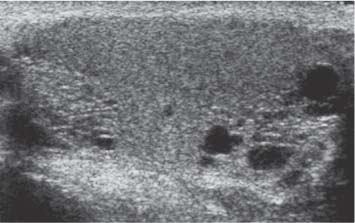
Rete Testis
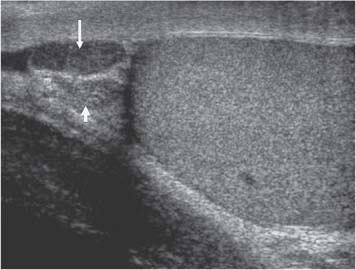
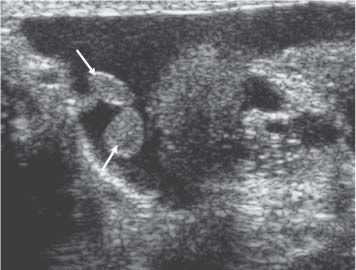
Appendix Testis
Polyorchidism
Intratesticular Focal Lesions
Testicular Tumors
Germ cell tumors |
Precursor lesions |
Intratubular germ cell neoplasia |
Tumors of one histological type |
Seminoma |
Embryonal carcinoma |
Yolk sac tumor |
Choriocarcinoma |
Teratoma |
Mature |
Immature |
With malignant transformation |
Tumors of more than one histological type |
Sex cordand stromal tumors |
Leydig cell tumor |
Sertoli cell tumor |
Granulosa cell tumor |
Fibroma-thecoma |
Tumors with both sex cord and stromal cells and germ cells |
Gonadoblastoma |
Lymphoid and hematopoietic tumors |
Lymphoma |
Leukemia |
Metastasis |
Most testicular tumors are of homogenous, low reflectivity in comparison to the surrounding testicular parenchyma, although a wide range of appearances occur, including high-reflective, heterogeneous lesions with areas of calcification and cystic change.20 Larger tumors demonstrate increased vascularity,21 although with the newer high-frequency transducers, malignant vascularity may be identified in small-volume tumors.22,23
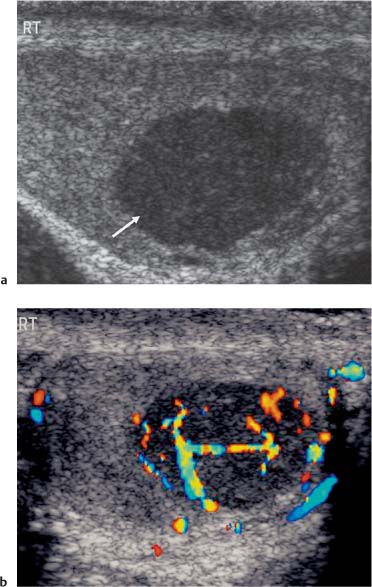
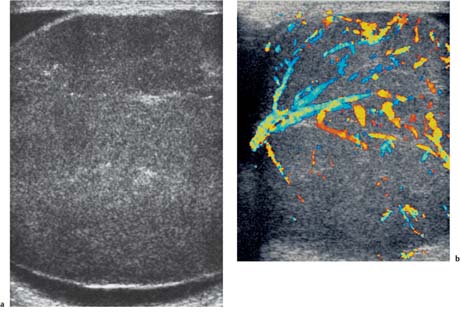
Fig. 11.15 a A low-reflective mass lying within testicular parenchyma, with a well-delineated border (arrow) demonstrating features of a seminoma. b Following the addition of color Doppler ultrasound, there is intralesional vascularity demonstrated in the seminoma
Summary points:
• Testicular tumors = 1% of all male neoplasm
• Higher incidence in the 15–34-year-old age group
• Increasing in prevalence for unknown reasons
• Cancer of white males
• Commonly presents as a painless scrotal mass
Germ Cell Tumors
The precursor of germ cell tumors is thought to be intratubular germ cell neoplasia; if development is along a “unipotential” gonadal line, a seminoma will form, but if development occurs along a “totipotential” gonadal line, a nonseminomatous tumor will develop.24The “totipotential” cells may remain undifferentiated (embryonal carcinoma), develop toward embryonic differentiation (teratoma) or extraembryonic differentiation (yolk sac tumors, choriocarcinoma). Multiple histological types occur together (mixed germ cell tumor), as these “totipotential” cells develop along multiple pathways.25
Most germ cell tumors spread via the lymphatic system rather than hematogenous, except for chorio-carcinoma. Normally testicular lymphatic drainage follows the testicular vein, occurring in a predictable pattern. Orchidectomy for all testicular tumors is performed through an inguinal approach to avoid skin involvement and spread to the external iliac nodes. Staging of testicular carcinoma follows the tumor, node, metastasis (TNM) classification.24 Tumor markers play an important role in diagnosis, staging, prognosis, and follow-up of germ cell tumors.26 The most important tumor markers are alpha-fetoprotein (AFP), human chorionic gonadotrophin (HCG), and lactate dehydrogenase (LDH).
Seminomatous Germ Cell Tumors
Seminoma is the most common pure germ cell tumor, accounting for up to 50% of cases, occurring in a slightly older patient group of age 40. The ultrasound appearances reflect the uniform cellular nature of the tumor; they are uniformly of low reflectivity, although larger tumors may be heterogeneous, lobulated, or present as multinodular areas in continuity (Fig. 11.15). These tumors are extremely radiosensitive.
Nonseminomatous Germ Cell Tumors
Mixed Germ Cell Tumors
Mixed germ cell tumor contains more than one germ cell component; any combination of cell type can occur. The average age of presentation is 30 years. These tumors constitute up to 60% of all germ cell tumors and are more common than the pure histological forms of testicular tumors (Fig. 11.16).
Embryonal Carcinoma
This is the second most common pure germ cell tumor after seminoma; embryonal carcinoma is present in 87% of mixed germ cell tumors, but in the pure form accounts for 2% of all testicular tumors. Embryonal carcinoma affects younger men (age 25–35) and tends to be more aggressive. These tumors are often heterogeneous, ill-defined, blending imperceptibly into adjacent testicular parenchyma.
Choriocarcinoma
Choriocarcinoma is a rare tumor, occurring in a pure form in < 1% of patients, but in a mixed germ cell tumor in 8% of patients, where it is highly malignant. Chorio-carcinoma carries the worst prognosis of any germ cell tumor; a high level of HCG confers a poor prognosis.
Yolk Sac Tumor
Yolk sac tumors account for 80% of childhood (< age two) tumors but is rare in adults. Elevation of AFP levels is present. The imaging features are nonspecific, often just testicular enlargement in children.
Teratoma
Teratoma is the second most common testicular tumor in children (< age four), and teratoma cells occur in over 50% of adult cases of mixed germ cell tumors. A teratoma tends to be a complex tumor, and the ultrasound features are those of a well-defined complex mass with cystic change (Fig. 11.17.). Calcification may be present. In the prepubertal testes a pure teratoma runs a benign course and testis-sparing surgery may be undertaken. This is not true for the postpubertal teratoma, which will metastasize irrespective of the histological features.
Epidermoid Cyst
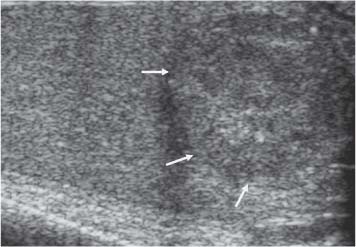
The pathogenesis of an epidermoid cyst is uncertain; it either arises from monodermal development of a teratoma or as a result of squamous metaplasia of surface mesothelium. These are benign lesions with no malignant potential.27 Epidermoid cysts comprise 1% of all testicular tumors and are true cysts, containing a cheesy laminated material. On ultrasound, epidermoid cysts are well-circumscribed with a high-reflective border and internal laminations giving an “onion-ring” appearance (Fig. 11.18).28
“Regressed” or “Burnt-out” Germ Cell Tumors
Patients may present with widespread metastases but no primary tumor except for an area of calcification within the testis.29 The pathogenesis of this phenomenon may be the result of a high metabolic rate of the tumor, which outgrows its blood supply and involutes (Fig. 11.19).
Fig. 11.16 A focal mass (arrows), a mixed germ cell tumor, is present at the lower aspect ofthe testis, heterogeneous but mainly high reflectivity and is not as clearly defined as the example of the seminoma in Fig. 11.15
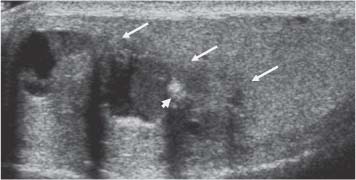
Fig. 11.17 A lobulated mass at the upper aspect of the testis (arrows) demonstrating cystic change and focal calcification (arrowhead). A teratoma
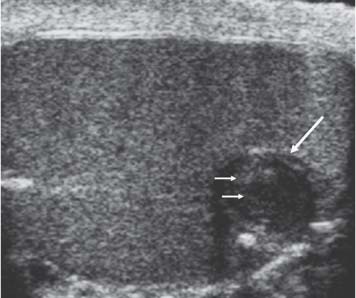
Fig. 11.18 An epidermoid cyst (long arrow). It demonstrates a well-circumscribed, low-reflective appearance with a high-reflective border and internal laminations (small arrows) giving an “onion-ring” appearance
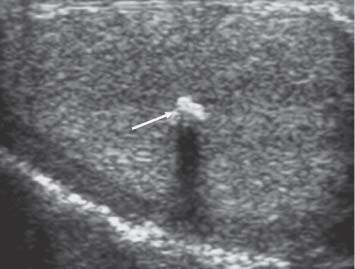
Fig. 11.19 A focal clump of calcification (arrow) is present in the central aspect of the testis on ultrasound in a patient with a retroperitoneal germ cell tumor. A “burnt-out” testicular tumor
Fig. 11.20 a A large, mixed-reflective, heterogeneous tumor at the lower aspect of the testis which on histological examination was found to be a Leydig cell tumor. b The same Leydig cell tumor demonstrating increased color Doppler flow in both a peripheral and central distribution
Summary points:
• Unipotential gonadal cell line develops into a seminoma
• Totipotential gonadal cell line develops into nonseminoma
• Multiple histological cell types occur together: Mixed germ cell tumor
• Tumor spread is via the lymphatic system
Nongerm Cell Tumors
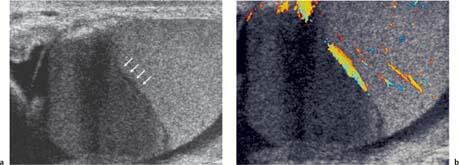
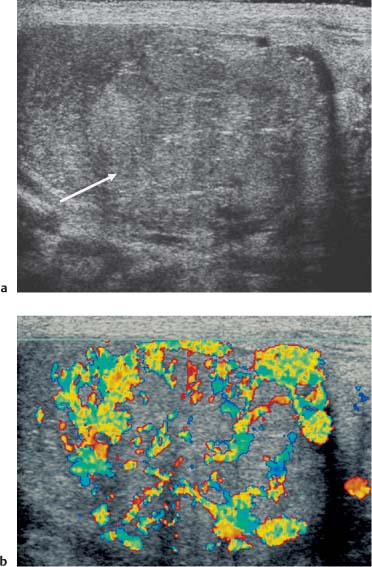
The prevalence of nongerm cell tumors is higher in the pediatric age group, constituting 30% of all testicular tumors. Nearly all nongerm cell tumors are benign, but there is no clear ultrasound criterion that allows differentiation from malignant testicular tumors. Leydig cell tumors occur across all age groups and account for 3% of all testicular tumors. Patients demonstrate symptoms related to androgen or estrogen secretion by the tumor, which includes precocious virilization, gynecomastia, or decreased libido. On ultrasound Leydig cell tumors are small, commonly of low reflectivity with cystic change.30 Use of color Doppler ultrasound may demonstrate poor internal color Doppler flow with increased peripheral vascularity in Leydig tumors when small, but vascularity increases with tumor size (Fig. 11.20).31 Sertoli cell tumors constitute 1% of all testicular tumors, and are less likely than Leydig cell tumors to secrete hormones. On ultrasound the Sertoli cell tumors are well-circumscribed, round, and lobulated.
Lymphoma
Testicular lymphoma may be the primary site of involvement, the initial manifestation of widespread disease, or the site of recurrence. Testicular lymphoma, which accounts for 5% of testicular tumors, is the most common testicular tumor in the over 60-year-old age group. The ultrasound appearances of testicular lymphoma are similar to germ cell tumors: Discrete, low-reflective lesions with increased color Doppler flow but complete testicular involvement may be seen, emphasizing the need to compare the reflectivity of both testes (Fig. 11.21).32
Leukemia
Primary leukemia of the testis is rare, although a common site of recurrence in children. The ultrasound appearances are very variable, being unilateral or bilateral, diffuse or focal, low- or high-reflective.33
Fig. 11.21 a There is enlargement of the testis, with a uniform low-reflective appearance with a surrounding hydrocele in a patient with testicular lymphoma. b Color Doppler ultrasound demonstrates a markedly abnormal vascular pattern to the lymphoma infiltrated testis
Metastasis
Metastasis to the testis is unusual, with the most frequent primary sites being the prostate, lung, melanoma, colon, and kidney.34 Metastasis usually occurs in advanced disease and is indistinguishable on ultra-sound from primary tumors of the testis (Fig. 11.22).
Splenogonadal Fusion
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree