Fig. 1
Elution profile of a 68Ge/68Ga generator where one fraction was 1 mL (fraction 1 = 0.3 mL, fraction 7 = 0.7 mL) for total eluted volume of 6 mL. The profiles for 68Ga elution and 68Ge breakthrough are similar. Fraction 3 (1 mL) contains over 60% of the available 68Ga activity. Inset: elution profile of 68Ga eluted from the small SAX SPE Chromafix cartridge with small volumes of deionized water. More than 90% of the initial 68Ga activity was obtained in 200 μL solution. Reproduced from Velikyan et al. (2004)
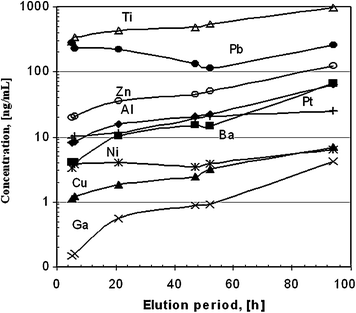
Fig. 2
Metal ion content in 6 mL generator eluate as a function of elution time period. Reproduced from Velikyan et al. (2004)
Buffers with weak complexing strength are used in labeling synthesis to keep 68Ga in solution, prevent precipitation and colloid formation, and provide pH required for formation of the desired complex. Use of 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) buffer resulted in preparation of tracers with higher specific radioactivity as compared with sodium acetate (Velikyan et al. 2004). Such buffers as succinate, formate, tris, glutamate, lactate, oxalate, and tartrate lead to poor radiochemical yields (Bauwens et al. 2010).
Labeling with high specific radioactivity (SRA) provides a wide range of SRA values that can be adjusted by addition of the cold vector molecule. This provides the possibility for optimization of the SRA for each application, considering the tracer affinity as well as the target expression level in both diseased and normal tissue. In targeted and pretargeted imaging, the requirement for sufficiently high SRA is dictated by biological factors such as the limited amount of binding sites, tracer affinity, and possible pharmacological side-effects. It might also be of interest to explore the influence of SRA on the quantification precision and the possibility for refined evaluation of receptor binding parameters, especially to determine in vivo the total receptor number in, e.g., tumors.
The radioactivity incorporation and thus the specific radioactivity of tracers can be enhanced using microwave heating and preconcentrated 68Ga (Velikyan et al. 2004; Velikyan 2005; Bergstrom et al. 2005; Blom et al. 2009; Lavén et al. 2004, 2005; Lendvai et al. 2005, 2008, 2009; Roivainen et al. 2004; Velikyan et al. 2004, 2008; Långström et al. 2008; Velikyan and Långström 2004; Velikyan et al. 2004, 2005; Tolmachev et al. 2010). Moreover, DOTA may have higher selectivity for Ga as compared with In and Fe under microwave heating conditions (Velikyan et al. 2008). The NOTA chelating moiety can be labeled at room temperature (Velikyan et al. 2008), providing mild reaction conditions for bioconjugates comprising temperature-sensitive, fragile macromolecules (Velikyan et al. 2008; Velikyan and Långström 2006; Riss et al. 2008).
Advancement in generator development and eluate quality enhancement methodologies in combination with macrocyclic chelators and most importantly the advent of receptor-targeted peptides, with the success story of SST, has accelerated applications involving 68Ga. Radiotracers for targeted imaging of specific protein expression products (receptors, enzymes, and antigens), pretargeted imaging using small effector or hapten molecules, as well as nontargeted imaging of pulmonary and myocardial perfusion and ventilation are being developed. Imaging agents to monitor inflammation and infection as well as general downstream biologic properties such as proliferation, hypoxia, glycolysis, and angiogenesis have also been investigated (Velikyan 2011). The variety of potential applications is analogous to that of 99mTc. In addition, 68Ga PET provides such benefits as higher sensitivity, resolution, quantitation, and dynamic scanning. Moreover, the similarity of the coordination chemistry of 68Ga to that of therapeutic radionuclides such as 90Y and 177Lu might allow for theranostics. The contribution of imaging diagnostics to cancer therapy in terms of early disease detection, staging, therapy selection, and follow-up is leading to personalized medicine.
The core of clinical nuclear medicine advancement is the development of new radiopharmaceuticals and their availability. The examples below demonstrate the diversity and range of potential 68Ga applications exemplified by targeted, pretargeted, and nontargeted imaging.
2 Targeted Imaging
Peptides labeled with a positron-emitting radionuclide can be used to make an accurate tumor diagnosis, determine staging, and quantify the radiation dose to tumors and critical organs, thus allowing dose planning and dose monitoring for successful radiotherapy, and to follow tumor response to chemo- and radiotherapy, thus providing personalized patient management (Lundqvist and Tolmachev 2002; Mutic et al. 2003; Kowalski et al. 2003; Baum et al. 2008; Reubi et al. 2005; Heppeler et al. 2000; Eisenwiener et al. 2002; Liu and Edwards 1999; Thakur 1995; Behr et al. 1999; Otte et al. 1997; de Jong et al. 1997; Schmitt et al. 2004; Pless et al. 2004; Meier et al. 2004; Tolmachev 1800; Maecke et al. 2005; Barone et al. 2005; Gambhir 2002; Carlsson et al. 2002; Tofilon et al. 2003; Carlsson et al. 2003; Forssell-Aronsson et al. 2002). The compatibility of 68Ga with peptides for targeted imaging of such receptors as somatostatin, bombesin, human epidermal growth factor, integrin, vascular endothelial growth factor, cholecystokinin-2, gastrin-releasing peptide, melanocyte stimulation hormone, glucagon-like peptide 1, gonadotropin-releasing hormone, folate, neurotensin, and neuropeptide Y receptors has been demonstrated (Tolmachev 1800; Maschauer et al. 2010; Mathias et al. 2003; Fani et al. 2011; Schottelius et al. 2008; Demmer et al. 2008; Brom et al. 2010; Wild et al. 2010; Froidevaux et al. 2004; Cantorias et al. 2009; Wei et al. 2007; Zhang et al. 2007; Schuhmacher et al. 2005; Dimitrakopoulou-Strauss et al. 2007; Hofmann et al. 2004; Baum et al. 2007; Cagnolini et al. 2010; Mansi et al. 2011; Reubi and Maecke 2008). The corresponding ligands commonly have satisfactory pharmacokinetics with fast blood clearance, relatively low hepatobiliary excretion, and mostly renal elimination as well as excellent tissue penetration, minimal side-effects, and no or low antigenicity.
2.1 Imaging Somatostatin Receptors
Somatostatin receptor (SSTR) ligand analogs are the most well-developed and extensively investigated group of small regulatory peptides. Somatostatin receptors are expressed in neuroendocrine tumors, renal cell carcinoma, small cell lung cancer, breast cancer, prostate cancer, and malignant lymphoma (Reubi 2001). The receptor binding affinity, internalization, and biodistribution of different SST ligands have been shown to be dependent on the peptide constitution, chelator type (1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA), desferrioxamine (DFO), diethylenetriaminepentaacetic acid (DTPA), and their derivatives), metal cations (In, Y, Ga, Tc, Lu), linkers, and pharmacokinetic modifiers (Kowalski et al. 2003; Eisenwiener et al. 2002; Maecke 2005; Rivier et al. 2005; Wild et al. 2003; 2005; Ginj et al. 2005; Hofland et al. 1999; De Jong et al. 1998; Reubi et al. 2000; Froidevaux and Eberle 2002; Forrer et al. 2004; Schmitt et al. 2005; Smith-Jones et al. 1994; Froidevaux et al. 2002; Heppeler et al. 1999; Henze et al. 2001; Deshmukh et al. 2005; Hofmann et al. 2001; Pettinato et al. 2008; Antunes et al. 2007; Erchegyi et al. 2008; Grace et al. 2008a, b; Cescato et al. 2008; Fani et al. 2010).
Quantification is one of the prominent advantages of the PET technique, allowing estimation of receptor density in vivo (Zhang et al. 2010). However, the accuracy and interpretation of results depend on a number of parameters, including SRA. The latter was demonstrated to be an essential tool for optimization of imaging quality and correlation of radioactivity uptake and receptor expression (Velikyan et al. 2008; Velikyan et al. 2010). In particular, the quantification accuracy of receptor binding of [68Ga]Ga-DOTA-TOC was investigated as a function of SRA in vitro on frozen sections of Rhesus monkey brain expressing SSTR. The dependence was described by a sigmoidal function with the region around the inflection point being the most sensitive to SRA variation (Fig. 3). The values of SRA corresponding to the top plateau provided stable target-to-nontarget ratio and thus high reproducibility of the in vitro biological assay and more accurate quantification (Velikyan et al. 2008). However, it should be mentioned that the correlation of the in vitro and in vivo uptake as a function of SRA might not be linear. Optimization of SRA is required, considering the losses of the tracer in vivo due to nonspecific adsorption, blood protein binding, and binding to physiologically expressed sites consuming tracer, thus demanding higher masses of vector and consequently lower SRA.
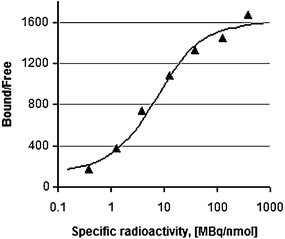

Fig. 3
Ratio of ligand bound to receptor and free ligand as a function of SRA, assuming constant radioactivity concentration. The ratio was obtained using the average of the uptake values for thalamus and cortex with relative standard deviation of 5–19%. The data were fitted to a sigmoid two-parameter Emax model. The optimization was done in MATLAB 7.0 using a least-squares method (LSQNONLIN, MATLAB, 2005, 7.0.4). Reproduced from Velikyan et al. (2008)
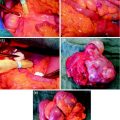
The short half-life of 68Ga, allowing repetitive sequential examinations, in combination with fast target localization and blood clearance of SST analogs is of special interest for clinical use, providing a means for individual patient treatment design (Maecke et al. 2005; Velikyan et al. 2010). Modulation of [68Ga]Ga-DOTA-TOC uptake in tumor and healthy organs was achieved by variation of the total amount of administered peptide. In particular, three sequential examinations of a patient were conducted on the same day, and the amount of octreotide administered immediately prior to [68Ga]Ga-DOTA-TOC during the second and third examinations was escalated (Velikyan et al. 2010). The uptake increased in the tumor and metastases and at the same time decreased in the liver and spleen upon injection of 50 μg. Further enhancement to 250 and 500 μg resulted in a blocking effect in all tissues (Fig. 4, row 1) except for one patient (Fig. 4, row 2). The latter observation clearly demonstrates the importance of individualized patient diagnostic and therapeutic management.
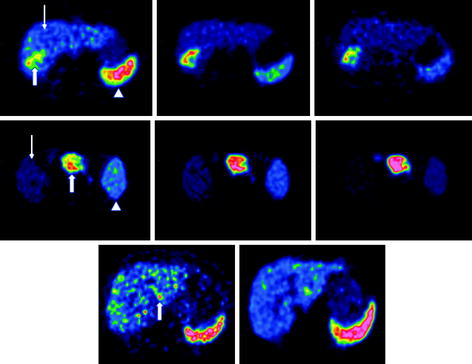

Fig. 4
Row 1 : Transaxial [68Ga]-DOTATOC-PET images of patient 1 with liver metastases from a colonic carcinoid who underwent three sequential PET-CT examinations. The tracer accumulation pattern in tumor tissue (thick arrow) increased in the second PET examination by pretreatment with 50 μg i.v. unlabeled octreotide but decreased again in the third examination that was proceeded by 500 μg i.v. octreotide. Row 2 : Transaxial [68Ga]-DOTATOC-PET images of patient 6 with a large endocrine pancreatic tumor who underwent three sequential PET-CT examinations. In contrast to the tumor uptake pattern in the other patients, as illustrated in row 1, the tumor accumulation (thick arrow) in this particular patient increased gradually over the three PET examinations. Row 3 : Transaxial [68Ga]-DOTATOC-PET images of patient 2, with multiple small liver metastases from a midgut carcinoid who was examined before and after interferon therapy. The [68Ga]-DOTATOC accumulation decreased in the tumor tissue (thick arrow) in the second PET examination performed after 3 months of interferon therapy. In all patients, the [68Ga]-DOTATOC PET accumulation in normal tissues, best appreciated in the spleen (arrow head) and liver (thin arrow), decreased gradually over the three examinations. Reproduced from Velikyan et al. (2010)
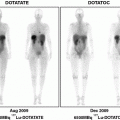
[68Ga]Ga-DOTA-TOC, [68Ga]Ga-DOTA-TATE, and [68Ga]Ga-DOTA-NOC have been extensively used in clinical studies, demonstrating fast pharmacokinetics, short scanning time, low radiation dose, and high sensitivity, resolution, detection rate, and image contrast, as well as the possibility for quantification (Kowalski et al. 2003; Baum et al. 2008; Henze et al. 2001; Hofmann et al. 2001; Velikyan et al. 2010; Hofmann et al. 2004; Koukouraki et al. 2006; Gabriel et al. 2007; Henze et al. 2004; Dimitrakopoulou-Strauss et al. 2006; Koukouraki et al. 2006; Kayani et al. 2008; Luboldt et al. 2010; Tan and Goh 2010; Ambrosini et al. 2010; Lincke et al. 2009; Rominger et al. 2010). Personalized diagnosis using these tracers is becoming a standard for selection of patient therapeutic management (Maecke et al. 2005; Henze et al. 2001; Gabriel et al. 2010; Putzer et al. 2010; Modlin et al. 2008; Win et al. 2007; Srirajaskanthan et al. 2010; Putzer et al. 2009; Milker-Zabel et al. 2006; Gehler et al. 2009; Prasad and Baum 2010; Ambrosini et al. 2010; Campana et al. 2010; Frilling et al. 2010; Versari et al. 2010; Van Riet et al. 2009; Ambrosini et al. 2008; Sainz-Esteban et al. 2010; Ambrosini et al. 2011; Traub-Weidinger et al. 2010; Boss et al. 2010; Henze et al. 2005; Conry et al. 2010). The vast experience of the clinical studies has been summarized in the form of guidelines for the assistance of nuclear medicine physicians in examination protocols as well as in interpretation and reporting of results (Virgolini et al. 2010; Janson et al. 2010).
2.2 Imaging Human Epidermal Growth Factor Receptor (HER) Family
Various 68Ga-labeled agents for imaging of the human epidermal growth factor receptor family overexpressed in malignant tumors such as breast, ovary, lung, colorectal, and urothelial have been developed and investigated (Yarden and Sliwkowski 2001; Walker and Dearing 1999; Witton et al. 2003; Hirsch et al. 2003; Neal and Mellon 1992; Vosjan et al. 2011). Thus, 53-amino-acid residue EGFR natural ligand (Velikyan et al. 2005; Sandstrom et al. 2011; Velikyan et al. 2006), 58-amino-acid residue Affibody® ligands (Tolmachev et al. 2010; Tolmachev and Orlova 2009; Ahlgren and Tolmachev 2010; Tolmachev et al. 2009), antibody fragments (Vosjan et al. 2011; Smith-Jones et al. 2004; Smith-Jones et al. 2006), two-helix Affibody derivative (Ren et al. 2009), as well as tyrosine kinase inhibitor (Theeraladanon et al. 2010) have been thoroughly evaluated in vitro and in vivo in xenograft animal models.
The ligand hEGF was conjugated with DOTA (Velikyan et al. 2005; Velikyan et al. 2006) and NOTA (Sandstrom et al. 2011), and the resulting conjugates were labeled with 68Ga and 67Ga, respectively, under microwave heating and room temperature. The biological activity maintenance of the tracers was thoroughly studied by performing cell-binding assays, biodistribution studies, and microPET imaging in tumor-bearing mice (Fig. 5, left). [68Ga]Ga-DOTA-hEGF demonstrated high affinity and fast pharmacokinetic (Fig. 5, right) compatible with application for in vivo imaging of breast or non-small cell lung cancer. However, imaging of head and neck squamous cell carcinoma would be challenging due to the high physiological expression of EGFRs particularly in salivary glands, thus resulting in high background uptake and masking of the signal from tumor cells. Blocking of uptake in healthy organs was necessary to decrease the background and improve the contrast. This could be achieved by pre-injection of nonlabeled EGF ligand. However, the required high amount of the latter would stimulate growth of cancer cells. To avoid the pharmacological effect, anti-EGFR Affibody® ligands [ZEGFR:1907, (ZEGFR:1907)2 or (ZEGFR:955)2] were used for saturation of the physiologically expressed EGFR prior to administration of the tracer molecule based on hEGF ligand ([67Ga]Ga-NOTA-Bn-NCS-hEGF) (Sandstrom et al. 2011). The dimeric Affibody molecule (ZEGFR:1907)2 exhibited the best results, and the tumor-to-organ ratio in liver, salivary glands, and colon was improved in nude mice bearing UTSCC-7 cell xenograft.
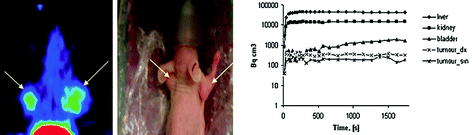

Fig. 5
(Left) Image showing a summation of frames 20–24 (20–30 min after injection) and photograph of the positioning of the mouse, in which the tumors (arrows) can clearly be seen at either side of the head. (Right) Pharmacokinetic curves showing the rapid distribution of [68Ga]Ga-DOTA-hEGF (0.16 nmol injected) to liver, kidney, and tumors. Tumor_dx = right-side tumor; Tumor_sin = left-side tumor. Reproduced from Velikyan et al. (2005)
68Ga-labeling of DOTA-conjugated Affibody® molecules can be accomplished using either conventional or microwave heating without compromising the receptor binding capability (Velikyan 2005; Tolmachev et al. 2010). The influence of radiometal exchange on the ligand in vivo distribution was studied in a dual experiment, allowing direct comparison of 68Ga- and 111In-labeled DOTA-ZHER2:342-pep2 (ABY-002). Co-injection of the tracers provided uniform conditions for the biology validation experiments, eliminated the variation that might originate from individual differences amongst animals, and decreased the use of animals. Both tracers demonstrated specific uptake in vivo in SKOV-3 xenografts in mice (Figs. 6, 7). However, due to the faster fade of the 68Ga-based counterpart from blood, lungs, gastrointestinal tract, and muscle, the tumor-to-organ ratio for this tracer was higher, thus resulting in better contrast (Tolmachev et al. 2010). Metastatic breast cancer was successfully visualized in clinical patient examination using [68Ga]Ga-ABY-002 (Baum et al. 2010). The agent was well tolerated and rapidly cleared from blood, providing high image contrast. This was found to be valuable specifically for HER2 status of metastases not amenable to biopsy. To accelerate clearance, the size of the Affibody® molecule was decreased to the two-helix molecule ([68Ga]Ga-DOTA-MUT-DS) (Ren et al. 2009). Although the clearance became faster, this modification resulted in a drop of the affinity and tumor uptake in SKOV3 xenografts.
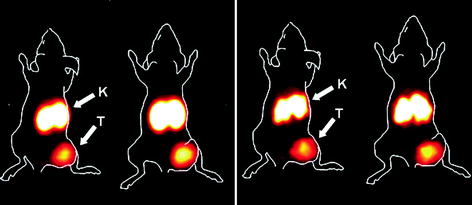
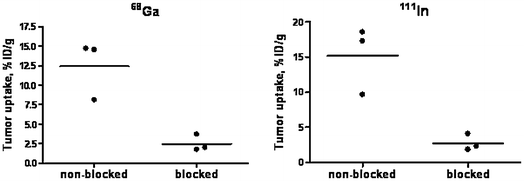

Fig. 6
Imaging of HER2 expression in SKOV-3 xenografts in BALB/c nu/nu mice using PET (left) and gamma-camera (right). Animals were injected with a mixture of 68Ga-DOTA-ZHER2:342 and 111In-DOTA-ZHER2:342. Animals were sacrificed 2 h p.i., and the PET image was acquired. Gamma-camera image was acquired 24 h later, after decay of 68Ga. To facilitate interpretation, animal contours were derived from a digital photograph and superimposed over the PET and gamma-camera image. Arrows indicate tumor (T) and kidneys (K) in one representative animal. Reproduced from Tolmachev et al. (2010)

Fig. 7
In vivo binding specificity of 68Ga-DOTA-ZHER2:342 (left panel) and 111In-DOTA-ZHER2:342 (right panel), 2 h p.i. The blocked group was subcutaneously pre-injected with an excess amount of nonlabeled ZHER2:342. Results are presented as percentage of injected dose per gram of tissue (% ID/g). Statistical significance in tumor uptake between the groups, according to Student’s t-test, gave p < 0.05. Reproduced from Tolmachev et al. (2010)
2.3 Imaging Angiogenesis
Imaging of integrin and vascular endothelial growth factor (VEGF) receptors overexpressed in tumors and ischemic injuries with extensive formation of new capillaries is an important means not only for diagnosis and monitoring response to therapy but also for antiangiogenic drug development. A ligand for VEGFR, single-chain VEGF (scVEGF), which is a functionally active single-chain version of VEGF (Backer et al. 2007, 2008), was conjugated to PEG linkers in order to adjust the pharmacokinetics and to couple to DOTA- or NOTA-based bifunctional chelators for efficient labeling with 68Ga (Blom et al. 2011). The chelators were conjugated to Cys-tag in scVEGF via bifunctional PEG linkers of various lengths (2.0, 3.4, and 5.0 kDa), and the impact of these modifications on the radiolabeling efficiency and functional activity of the ligand was assessed. The labeling efficiency did not depend on the PEG linker length and was comparable for both DOTA and NOTA chelating moiety at elevated temperature provided by either conventional or microwave heating. However, NOTA offered the additional advantage of labeling at ambient temperature, providing mild labeling conditions for the relatively large sc-VEGF (~28 kDa) and the possibility for cold kit-type tracer production. An in vitro binding assay using multicellular spheroids of 293/KDR cell line confirmed the biological functionality of the scVEGF-based tracers. Accumulation of [68Ga]Ga-NOTA-PEG-scVEGF could be detected in vivo in mouse 293/KDR xenografts. High kidney uptake was characteristic for all analogs.
An extensive number of peptide ligands with an exposed arginine-glycine-aspartic acid (RGD) sequence for binding to αvβ3 integrin receptors have been developed during the last two decades with the objective of visualizing angiogenesis and improving related drug development, diagnosis, and therapy monitoring (Dijkgraaf and Boerman 2009; Bergstrom et al. 2003; Garner and Lappin 2006; Chen et al. 2004; Haubner et al. 2004; Janssen et al. 2002; Liu 2006; Decristoforo et al. 2008; Indrevoll et al. 2006; Haukkala et al. 2009; Liu et al. 2009; Jeong et al. 2008; Li et al. 2008; Dijkgraaf et al. 2011). Specific radioactivity of a DOTA-containing analog could be increased considerably using microwave heating (Velikyan et al. 2004; Långström et al. 2008). Two bioconjugates with the same constitution but dislocation of a few amino acids with the aim of creating RGD sequence in one counterpart and DGF sequence in the other (negative control) were designed with the aim of demonstrating the necessity of the RGD motif for specific binding to αvβ3 integrin receptors. The distribution of these analogs was studied in primates using PET/CT imaging (unpublished data). Increased accumulation of the RGD counterpart was detected in the wall of the uterus, probably indicating neovascularization, and most importantly it could partly be blocked by co-injection of excess cold analog, indicating binding specificity. Uptake of the analogs in other tissues was low.
2.4 Antisense Oligonucleotide-Based Tracers
Another class of biological macromolecules for targeted imaging is antisense oligonucleotides, which can be considered for in vivo imaging of gene expression and tumors that express ras oncogene point mutations (Tavitian 2005; Lendvai et al. 2009). An antisense oligonucleotide is a short, synthetic nucleic acid that may selectively hybridize with its complementary sequence in messenger RNA (mRNA). A number of various analogs with modifications in backbone, furanose ring moiety, as well as 3′- and 5′-end nucleotides for subsequent conjugation with bifunctional chelators were designed (Velikyan 2005; Lendvai et al. 2005, 2008; Velikyan et al. 2004). Labeling of oligonucleotides up to 9.8 kDa with 68Ga was performed under microwave heating. Tracers based on phosphodiester, phosphorothioate, 2′-O-methyl phosphodiester, locked nucleic acid (LNA), LNA-DNA mixmer (LNA and DNA nucleotides in alternation along the sequence), and peptide nucleic acid were thoroughly assessed in vitro and in vivo in rat (Lendvai et al. 2005, 2008, 2009; Roivainen et al. 2004; Velikyan et al. 2004). The hybridization capability of the analogs was controlled in situ, where the concentration of the 68Ga-labeled antisense oligonucleotide was kept constant while the concentration of sense was gradually increased, resulting in a gradual increase of hybrid formation (Fig. 8). The radioactivity organ distribution pattern varied considerably depending on the oligonucleotide modification. Uptake in nonhybridization specific tissue was most probably mediated by scavenger receptors (Lendvai et al. 2009). In vivo tumor accumulation of 68Ga-labeled 17-mer antisense phosphorothioate oligonucleotide was observed in rat model bearing xenografts with activated K-ras point mutation mRNA (Fig. 9) (Roivainen et al. 2004). Radioactivity accumulation was not detected in the negative control.
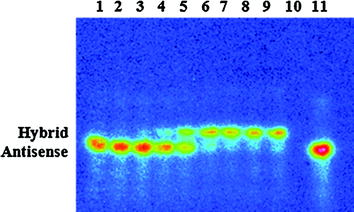
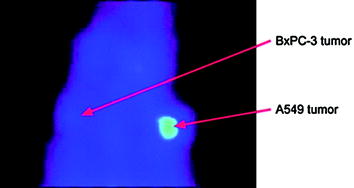

Fig. 8
Autoradiography of the polyacrylamide gel. Concentration-dependent hybridization of 68Ga-labeled 17-mer antisense phosphorothioate oligonucleotide to the complementary 17-mer sense phosphodiester oligonucleotide in solution. The sense concentration was gradually increased (lanes 1–9); the references are sense oligonucleotide (lane 10), 68Ga-labeled antisense oligonucleotide (lane 11), and molecular weight marker (lane 12)

Fig. 9
PET image of an athymic rat bearing both A549 and BxPC-3 tumors, using 68Ga-labeled PS antisense oligonucleotide specific to codon 12 mutated human K-ras oncogene. The human A549 cells (lung adenocarcinoma) contain K-ras point mutation in codon 12, and the human BxPC-3 cells (pancreatic adenocarcinoma, negative control) contain wild-type K-ras oncogene. Reproduced from Roivainen et al. (2004)
3 Pretargeted Imaging
The pretargeting concept has been introduced in order to overcome the problem in radioimmunotherapy and diagnostic imaging related to the slow pharmacokinetics and clearance of antibodies, leading, respectively, to high radiation dose to healthy tissue and poor image contrast (Hong et al. 2008; Frangioni 2009; Chang et al. 2002; Sharkey et al. 2010a, b; Goldenberg et al. 2006; Boerman et al. 2003). In the pretargeting process, bispecific antibody (bsmAb) or protein is first administered and given time for target localization and clearance from blood and healthy tissue. In the second step, a radiolabeled hapten or effector molecule with high affinity to the bispecific antibody or protein as well as fast pharmacokinetic and clearance properties is administered.
A bsmAb consists of two antigen-specific F(ab’)2 fragments covalently linked to a different F(ab) fragment for reaction with a hapten molecule (Fig. 10a). Various bsmAb constructs with mono- or divalent binding to tumor antigen as well as mono- and bivalent hapten molecules for imaging and therapy of breast cancer (anti-MUC1) (Schuhmacher et al. 2001a, b), melanoma (anti p97) (Somasundaram et al. 1993), lymphoma (anti-CD20, anti-HLA-DR) (Rossi et al. 2010; Sharkey et al. 2008), colorectal (anti-CEA) (Griffiths et al. 2004; Watine et al. 2001; Goldstein and Mitchell 2005), and pancreatic (anti-MUC1) (Gold et al. 2008; Forster et al. 2006) cancers have been investigated (Gold et al. 2008). The first pretargeting step may require several days, while the second step of radiolabeled hapten molecule administration and imaging may be accomplished within 1 h. A number of bivalent peptide hapten molecules containing histamine-succinyl-glycine (HSG) residues and coupled to a chelate moiety (Fig. 10b) have been developed and used in combination with bsmAb specific to carcinoembryonic antigen (Griffiths et al. 2004; Sharkey et al. 2005; Goldenberg and Sharkey 2007; Goldenberg et al. 2008). Preclinical assessment in mice bearing human colon tumor xenografts revealed the superiority of pretargeted imaging using 67Ga/68Ga-labeled hapten peptide as compared with that of [18F]-FDG in terms of uptake specificity and sensitivity (Schoffelen et al. 2010).
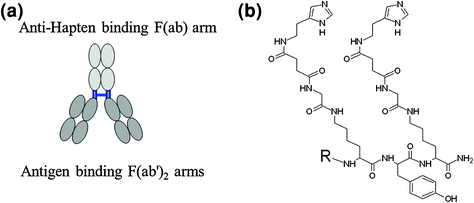

Fig. 10
a Schematic presentation of bsmAb. b Hapten molecule containing histamine-succinyl-glycine (HSG) residues, where R stands for the DOTA- or NOTA-based chelate moiety
High affinity of an effector molecule such as biotin for avidin and streptavidin has also been employed in the pretargeting technique. In this setting, the target is first hit with a corresponding ligand, for example, antibody fragment (Forster et al. 2006) modified with avidin or streptavidin, then visualized by subsequent introduction of radiolabeled biotin.
Pretargeting can be utilized not only for imaging and therapy of cancer. It has also been explored to monitor the migration and survival of cells after transplantation of islets of Langerhans for treatment of type 1 diabetes mellitus. The influence of various modifications such as chelate moiety and linker on the binding capability of biotin to avidin was investigated (Blom et al. 2009). Introduction of DOTA and PEG or (CH2)5 chains (Fig. 11) did not deteriorate the binding specificity, but slightly decreased the binding efficiency as compared with the native biotin. The analog, [68Ga]Ga-DOTA-(PEG)2-biotin, demonstrated the highest stability in human serum, and its complex with avidin was even more stable (80% intact during 120 min). This tracer was further assessed preclinically using avidin-coated agarose resin microbeads (AARs) mimicking islets and avidin-coated islets of Langerhans isolated from human pancreata (Eriksson et al. 2011). The binding of the tracer to both systems was specific as confirmed by uptake blocking using excess native biotin in vitro. The tracer alone demonstrated fast distribution in vivo in mice, dominated by the partially blockable liver uptake and renal clearance. AARs were transplantated intraportally into diabetic C57BL/6 mice and showed increased uptake of the tracer in the liver as compared with baseline. These preliminary and promising results demonstrated that [68Ga]Ga-DOTA-(PEG)2-biotin has potential to become an imaging agent for monitoring survival of transplanted islets.
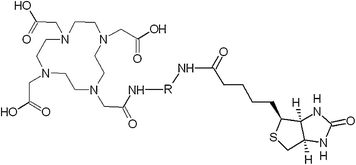

Fig. 11
Structure of biotin conjugate with DOTA moiety linked via spacer. 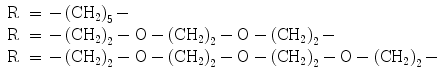
4 Nontargeted Imaging
Particle-based imaging agents have been developed to deliver imaging reporters or therapeutic agents to the target, to assess blood flow and lung ventilation. Extensive work has been conducted on 68Ga-labeling and preclinical assessment of nontoxic, nonantigenic, and biodegradable human serum albumin microspheres (HSAM), macroaggregated albumin (MAA), and albumin nanoparticles for examination of lung function by measuring pulmonary perfusion, cerebral blood flow, myocardial perfusion, vascular permeability, tumor perfusion, and blood pool by measuring angiogenesis as well as imaging and localization of sentinel lymph nodes for diagnosis, prognosis, as well as therapy and surgery planning (Schuster 1998; Wagner and Welch 1979; Yvert et al. 1979; Hayes et al. 1981; Steinling et al. 1985; Chesler et al. 1975; Mintun et al. 1986; Even and Green 1989; Mathias and Green 2008; Maziere et al. 1986; Maus et al. 2011; Wunderlich et al. 2010; Schiller et al. 2008; Richter et al. 2010; Beller et al. 1979; Schmidt et al. 2006; Hoffend et al. 2005; Mier et al. 2005; Schuster et al. 1995).
68Ga-labeled carbon nanoparticle aerosol was developed for diagnosis of pulmonary emboli and assessment of lung ventilation distribution prior to lung resection. The agent was prepared using commercially available equipment (Technegas, Australia) wherein preconcentrated 68Ga (Velikyan et al. 2004) was placed in a carbon crucible and exposed to high temperature, forming a pseudogas for inhalation (GallGas) (Borges et al. 2011). A comparative dual study was conducted in piglets wherein both [68Ga]Ga-GallGas and [99mTc]-Technegas were inhaled by the same animal. There was equally homogeneous distribution of radioactivity in healthy control piglets (Fig. 12a). Absence of radioactivity and ventilation in obstructed lower lobe could be observed in both PET and SPECT images (Fig. 12b). However, the former exhibited larger variation in ventilation distribution, most probably related to diffuse bronchial constriction. The most prominent difference in the performance of the two imaging agents was observed in piglets with diffuse airway obstruction induced by infusion of methacholine (Fig. 12c). Poorly and well-ventilated regions could be distinguished by PET agent and correlated with measurement of end-expiratory lung volume by multiple-breath nitrogen washout. The superior performance of [68Ga]Ga-GallGas in terms of better appreciation of heterogenecity of the airway response to bronchoconstrictive challenge was assigned to the higher resolution of PET as compared with SPECT. Distribution of similar 68Ga-labeled aerosol was studied in two healthy volunteers (Kotzerke et al. 2010). Even uptake of radioactivity was observed in lung and without bronchial deposits. After inhalation, the radioactivity remained in the alveolar space, and no elimination via blood, urine, or feces could be observed within 3.5 h.
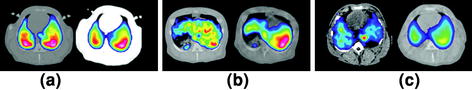

Fig. 12
Lung ventilation imaging using carbon particles behaving as a “pseudogas” for diagnosis of acute pulmonary emboli. Dual study using [68Ga]Ga-GallGas (left) and [99mTc]-Technegas (right) in healthy piglets, demonstrating similarity in ventilation distribution (a), in piglets with lower lobar obstruction (b), and in piglets with diffuse airway obstruction induced by infusion of methacholine (c). (Borges et al. 2011)
5 Low-Molecular-Weight Imaging Agents
Not only macromolecules have been tagged with 68Ga but also small biologically active ones for visualization of cell proliferation, hypoxia, glycolysis, myocardium, and bone. Analogs comprising nitroimidazole and mercaptobenzynamine have been investigated for imaging of hypoxia, which is an important parameter in tumor and myocardial ischemia physiology (Chapman et al. 1983; Hoigebazar et al. 2010; Mukai et al. 2009). Detection of hypoxia is important for diagnosis, prognosis, treatment planning, and monitoring response to therapy. Lipophilic and cationic gallium complexes of N4O2, N2S2, and NS3 have demonstrated myocardial uptake and retention in animal studies (Yang et al. 2010; Tsang et al. 1993; Hsiao et al. 2009; Plossl et al. 2008; Cutler et al. 1999; Zhang et al. 1992). Biphosphonates (Fellner et al. 2010) and 68Ga-labeled ethylene diamino-N,N,N’,N’-tetrakis-methylene phosphoric acid (Toegel et al. 2008; Mitterhauser et al. 2007) were found useful for early diagnosis of bone metastases. DOTA- and NOTA-coupled alanine and lysine analogs targeting transporters for cell proliferation visualization showed uptake in animal tumor models (Shetty et al. 2010a, b). [68Ga]Ga-EC-guanine (Yang et al. 2006) and [68Ga]Ga-EC-deoxyglucose (Yang and Kim 2005; Yang et al. 2005) have also demonstrated tumor uptake in animals.
6 Further Examples of Diverse Applications of 68Ga
The list of examples of diverse applications of 68Ga is rather extensive. 68Ga-labeling of high-affinity urea-based inhibitors of prostate-specific membrane antigen (PSMA) for imaging of prostate cancer is an example of the translation of the 99mTc heritage to 68Ga. N,N’-bis(diethylenetriamine pentaacetic acid)-pamoic acid bis-hydrazide was labeled with 68Ga for visualization of necrosis that occurs in acute myocardial infarction, chronic heart failure, allograft rejection, stroke, neurodegenerative disorders, and inflammation (Prinsen et al. 2010). Substrates for overexpressed multidrug-resistance P-glycoprotein were developed for imaging and evaluation of tumor response to therapy (Sharma et al. 2005; Thews et al. 2010). 68Ga-labeled ligand to vascular adhesion protein-1 (Lankinen et al. 2008; Autio et al. 2010; Silvola et al. 2010; Ujula et al. 2009), ionic 68Ga (Makinen et al. 2005), 68Ga-labeled siderophores (Petrik et al. 2010), and [68Ga]Ga-citrate (Rizzello et al. 2009; Nanni et al. 2010) have been suggested for differentiation and diagnosis of inflammation and infection. Imaging of thrombosis and atherosclerosis (Welch et al. 1977; Yano et al. 1985) and pulmonary function (Mintun et al. 1987; Schuster et al. 2002) are further examples.
7 Conclusions
The diversity of 68Ga-based imaging agents covers a broad range of vectors from small molecules to macromolecules, and further to particles. The advent of macrocyclic chelators, availability of 68Ge/68Ga generators, development of methods for 68Ga preconcentration and purification, and breakthrough experience with somatostatin analogs have considerably contributed to the exponential growth of 68Ga applications. The heritage of 99mTc plays an important role and influences the expansion of the 68Ga-based tracer arsenal. 68Ga-labeling chemistry is amenable to kit-type as well as automated radiopharmaceutical production, and is becoming a cost-effective complement to cyclotron-based tracers. It has the potential to facilitate development of clinically practical PET and may promote the PET technique worldwide for earlier and better diagnostics and beyond towards individualized medicine.
References
Ahlgren S, Tolmachev V (2010) Radionuclide molecular imaging using affibody molecules. Curr Pharm Biotechnol 11(6):581–589PubMed
Ambrosini V, Tomassetti P, Castellucci P, Campana D, Montini G, Rubello D, Nanni C, Rizzello A, Franchi R, Fanti S (2008) Comparison between 68 Ga-DOTA-NOC and 18F-DOPA PET for the detection of gastro-entero-pancreatic and lung neuro-endocrine tumours. Eur J Nucl Med Mol Imaging 35(8):1431PubMed
Ambrosini V, Zompatori M, De Luca F, Antonia DE, Allegri V, Nanni C, Malvi D, Tonveronachi E, Fasano L, Fabbri M, Fanti S (2010a) 68 Ga-DOTANOC PET/CT allows somatostatin receptor imaging in idiopathic pulmonary fibrosis: preliminary results. J Nucl Med 51(12):1950–1955PubMed
Ambrosini V, Nanni C, Zompatori M, Campana D, Tomassetti P, Castellucci P, Allegri V, Rubello D, Montini G, Franchi R, Fanti S (2010b) Ga-68-DOTA-NOC PET/CT in comparison with CT for the detection of bone metastasis in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging 37(4):722–727PubMed
Ambrosini V, Campana D, Allegri V, Opocher G, Fanti S (2011) 68 Ga-DOTA-NOC PET/CT detects somatostatin receptors expression in von Hippel-Lindau cerebellar disease. Clin Nucl Med 36(1):64–65PubMed
Antunes P, Ginj M, Walter MA, Chen J, Reubi JC, Maecke HR (2007) Influence of different spacers on the biological profile of a DOTA-somatostatin analogue. Bioconjugate Chem 18(1):84–92
Asti M, De Pietri G, Fraternali A, Grassi E, Sghedoni R, Floroni F, Roesch F, Versari A, Salvo D (2008) Validation of Ge-68/Ga-68 generator processing by chemical purification for routine clinical application of Ga-68-DOTATOC. Nucl Med Biol 35(6):721–724PubMed
Autio A, Ujula T, Luoto P, Salomaki S, Jalkanen S, Roivainen A (2010) PET imaging of inflammation and adenocarcinoma xenografts using vascular adhesion protein 1 targeting peptide 68 Ga-DOTAVAP-P1: comparison with 18F-FDG. Eur J Nucl Med Mol Imaging 37(10):1918–1925PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree