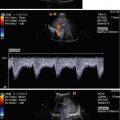
Fig. 13.1
Schematic drawing of the scrotal contents (Courtesy of Dr. Felix Trinkler, Zürich, Switzerland, and Deutscher Ärzte-Verlag GmbH). (Drawing by Blankvisual, Thun, Switzerland)
Three major atrial appendages have been described: the appendix testis, the appendix epididymitis and the appendix vas aberrans. The different appendices are remnants of embryonic structures. The appendix testis is a remnant of the müllerian duct, and the appendix epididymitis is a remnant of the mesonephron. Only the appendix testis and epididymitis can be displayed with ultrasound. This is very easy if fluid surrounds the testis (hydrocele). In normal individuals, the different atrial appendices cannot always be displayed. The appendix testis (hydatid of Morgagni) is attached to the upper pole of the testis in the groove between the testis and the epididymis (Dogra et al. 2003). The appendix epididymis is attached to the head of the epididymis.
The spermatic cord contains the ductus deferens, the cremasteric, ductus deferential and testicular artery and the plexus pampiniformis. It enters the internal inguinal ring, passes the inguinal canal and runs from the external inguinal ring to the posterior aspect of the testis.
13.2 Arterial Perfusion
Three arteries are responsible for the perfusion of the testis: the testicular, cremasteric and deferential arteries (Fig. 13.2a, b). These three arteries are connected by several anastomoses (Fig. 13.2a, b).
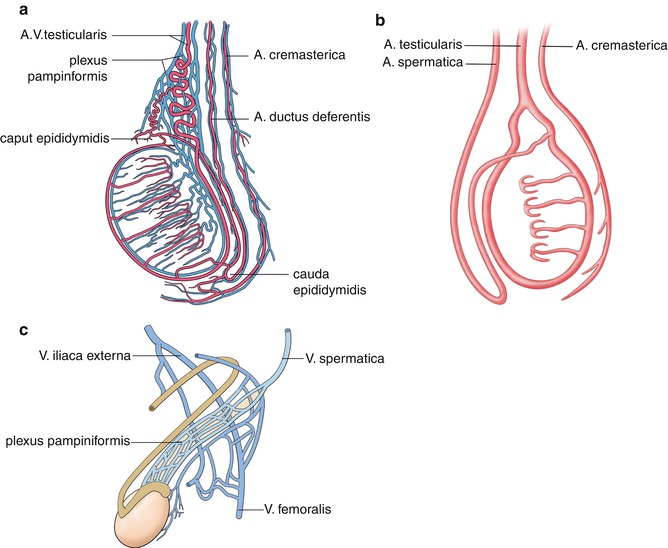

Fig. 13.2
(a) Arterial perfusion of the testis. The testis is perfused by the testicular, cremasteric and deferential arteries (Courtesy of Dr. Felix Trinkler, Zürich, Switzerland, and Deutscher Ärzte-Verlag GmbH). (b) Schematic drawing of the main arteries which perfuse the testes. There are multiple anastomoses between the different arteries. (c) Venous drainage of the testes by the plexus pampiniformis which drains into the spermatic vein and the external iliac vein (Courtesy of Dr. Felix Trinkler, Zürich, Switzerland, and Deutscher Ärzte-Verlag GmbH). (Drawing by Blankvisual, Thun, Switzerland)
The testicular artery originates from the abdominal aorta just beneath the renal arteries. It enters the testis in the region of the mediastinum testis.
The deferential artery originates from the superior or inferior vesical artery. It perfuses the ductus deferens and the cauda of the epididymis testis. In the region of the cauda, anastomoses with the testicular artery exist.
The cremasteric artery originates from the inferior epigastric artery and perfuses the spermatic cord. In the region of the mediastinum testis anastomoses between the other two arteries exist. Additionally anastomoses with the arteries of the scrotal wall can be found.
The deferential and cremasteric arteries supply the epididymis, vas deferens and peritesticular tissue.
Partial occlusion of one artery may partially be compensated by the collateral flow of the other arteries. This is very important for the diagnosis of torsion of the spermatic cord.
The right and left testicular arteries are the primary vascular supply to the testes. They enter the spermatic cord at the inner inguinal ring and continue along the posterior aspect of the testis, penetrating the tunica albuginea. The centripetal arteries arise from the capsular arteries and carry blood to the mediastinum, where they divide to form the recurrent rami that carry blood away from the mediastinum into the testis (Fig. 13.2a, b) (Dogra et al. 2003).
13.3 Venous Drainage
Several small veins originate from the mediastinum testis and form the plexus pampiniformis (Fig. 13.2c). Three groups of veins with many confluences form the plexus pampiniformis: The anterior group of the testicular vein drains foremost the testis, the middle group of the testicular vein drains especially the ductus deferens and the posterior group the cremasteric vein. All three veins have a lot of communications forming the plexus pampiniformis. The ductus deferential vein drains into the veins of the pelvis, whereas the cremasteric vein flows into the vena epigastrica inferior superficialis and profunda and the vena pudenda externa and interna. Besides the main drainage by the testicular vein, many collateral veins with the deferential vein and the cremasteric vein exist.
The right testicular vein flows into the inferior vena cava just beneath the right renal vein. The left testicular vein flows into the left renal vein in an angle of 90°. This is the reason why 90 % of all varicoceles occur on the left side.
13.4 Technical Prerequisites
For scrotal sonography a high-resolution linear transducer should be used. The transducer should have a length of 4–5 cm and a transmission frequency of at least 10 MHz (Fig. 13.3). Higher frequencies allow better detail resolution but have lower penetration.


Fig. 13.3
Linear probes for the Doppler sonographic investigation of the scrotum with 15 MHz and 8 MHz
The testes are examined in at least two planes, along the long and transverse axes. The size and echogenicity of both testes and epididymites are compared with the opposite side (Fig. 13.4a, c).
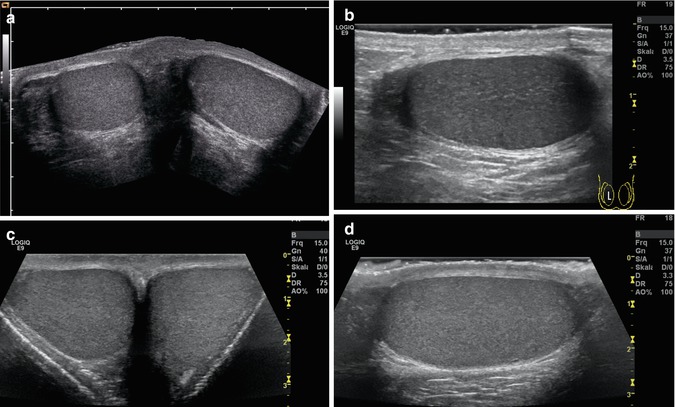

Fig. 13.4
(a, b) Panoramic transverse view of the normal testes of a 12-year-old boy (a) and a 19-year-old young man (c). The testes have a fine homogeneous echogenicity. Both testes are separated by a midline septum (c). (b, d) Longitudinal view of the normal testis of an 11-year-old boy (b) and a 19-year-old young man (d). On the left side of the image, the head of the epididymis is displayed
For the differential diagnosis of the acute scrotum, side-by-side investigation and the different Doppler techniques are mandatory. The Doppler device should be set as sensitive as possible, to detect the low flow within the testis. The lowest wall filter (<100 Hz) and the lowest pulse repetition frequency and Nyquist limit (1–2 cm/s) should be chosen. Power Doppler is helpful for the display of intratesticular flow in patients with acute scrotum (Bader et al. 1997; Barth and Shortliffe 1997; Hendrikx et al. 1997; Lee et al. 1996; Luker and Siegel 1996; Rubin et al. 1994; Sparano et al. 2008). Some authors state that power Doppler improves the viability of intrascrotal vessels in patients with acute scrotum (Bader et al. 1997; Barth and Shortliffe 1997; Luker and Siegel 1996; Rubin et al. 1994).
The prerequisites for scrotal sonography are summarised in Table 13.1.
Table 13.1
Prerequisites for scrotal sonography
Linear transducer with a length of ≥4 cm |
Transmission frequency ≥10 MHz |
Possibility of colour and pulsed Doppler sonography |
Low wall filter (<100 Hz) |
Low pulse repetition frequency (1–2 KHz) |
Spectral Doppler |
In all patients with suspected testicular torsion or other forms of acute scrotum, and in patients after scrotal trauma, pulsed Doppler sonography has to be performed additionally to colour Doppler. The comparison of colour Doppler, especially power Doppler, and scintigraphy showed excellent correlation between both methods (Luker and Siegel 1994; Middleton et al. 1990).
In patients with an acute scrotum, the initial investigation should begin with the healthy, painless testis. Ultrasound of the normal testis is used for the adjustment of adequate flow settings to show vessels within the testis. Optimal settings of the machine include a low wall filter (<100 Hz), low pulse repetition (1–2 KHz) frequency and gain setting. Due to the low flow within the testis, high gain settings are necessary. The best method is to increase the gain till flow artefacts appear, and then decrease the gain down slightly. If the flow in the normal testis can be shown without flow artefacts, the affected hurting testis can be investigated afterwards.
In infants with a small scrotum, the investigation can be performed without additional preparations. In older boys and in adolescents, a towel should be placed under the scrotum to elevate both testes. In the latter group the penis should be placed on the lower abdomen, wrapped and held by a towel to prevent psychological discomfort.
13.5 Sonographic Anatomy
The 2D image shows the testis as an oval-shaped organ with homogeneous echogenicity (Fig. 13.4). With increasing age, the echogenicity increases with puberty and adolescence. The testis has a smooth outer border without a visible capsule. In patients with a hydrocele, however, the capsule of the testis, the tunica albuginea, can be shown (Fig. 13.5a). The mediastinum testis runs as an echogenic band along the outer margin of the testis. The epididymis is slightly more echogenic and coarse in texture than the testis (Fig. 13.7a) (Ingram and Hollman 1994). The size of the testis increases with age. In adolescents and adults the testis measures between 3 and 5 cm in length and 2–3 cm in width (Zwiebel et al. 2005). High-resolution linear transducers with a frequency over 14 MHz may display the normal lobulation of the testes (Fig. 13.5).


Fig. 13.5
(a, b) High-resolution sonogram of a 16-year-old boy shows normal lobulation of the testicular tissue (Courtesy of Dr. Andreas Lemmer, Erfurt, Germany)
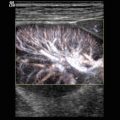
With colour-coded and power Doppler sonography, blood vessels can easily be shown in and around the testis in schoolboys, adolescents and adults (Fig. 13.6). If sensitive flow settings are used, even in infants vascularisation of the scrotal contents can be displayed.


Fig. 13.6
Colour-coded Doppler sonogram of the vessels in normal testes of adolescents. (a) Longitudinal view of the perfusion of the testis and the head of the epididymis. (b) Longitudinal view of the flow in the body of the epididymis and testis of a 19-year-old man
In all patients with scrotal pain the spermatic cord should be visualised (Figs. 13.7 and 13.8). The spermatic cord enters the internal inguinal ring, which has a diameter <4 mm. It courses through the inguinal canal, leaves the external inguinal ring and runs in a straight course to the cranial and posterior aspect of the epididymis and testis (Fig. 13.7b). There is no abrupt change in the course, size and shape of the spermatic cord (Fig. 13.7b). The diameter of the spermatic cord in healthy children never exceeds 10 mm (Baud et al. 1998). The spermatic cord can clearly be seen as a straight tubular structure, slightly medial to the head of the epididymis. It is displayed as multiple well-defined linear hypoechoic lines which are surrounded by echogenic walls (Fig. 13.7a–c). The linear structures are formed by the ductus deferens, the arteries and veins of the plexus pampiniformis. Without colour Doppler and spectral Doppler, differentiation between the various tubular structures is not possible (Fig. 13.7d).



Fig. 13.7
Longitudinal section through the spermatic cord. (a–c) 2D image; (d) colour Doppler imaging. (a) Longitudinal section through the groin and the upper part of the scrotum shows the testis and epididymis on the right side of the image. On the left side several linear structures are displayed, which are the ductus deferens and the vessels of the spermatic cord. (b, c) Longitudinal section through the groin shows multiple linear tubular structures caused by the various elements of the spermatic cord. (d) Colour Doppler sonogram of the flow within the vessels of the spermatic cord. The vessels run gyrose

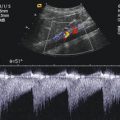
Fig. 13.8
Transverse section through the spermatic cord in the region of the groin with a high-resolution linear transducer. (a) 2D image. (b) Colour Doppler of the transverse section through the spermatic cord. CA cremasteric artery, DA deferential artery, DD ductus deferens, TA testicular artery, V draining vein
Transverse images show several round or oval-shaped anechoic or hypoechoic structures caused by the arteries, veins and the ductus deferens (Fig. 13.8a). Each vessel keeps the same position in the cord. The different Doppler techniques make the differentiation possible (Fig. 13.8b). Transverse images display the vessels as colour spots and not as loops (Baud et al. 1998).
Longitudinal sections of the upper border of the testis show the junction of the spermatic cord with the testis and the course of the testicular vessels at the dorsal aspect of the testis (Fig. 13.9).


Fig. 13.9
(a, b) Colour Doppler sonogram of the vessels of the testis, epididymis and the testicular cord. The vessels are displayed at the posterior aspect of the testis and leave the testis and epididymis at the cranial aspect of the testes where they form together with the ductus deferens (not displayed) the spermatic cord
Colour Doppler of the testis displays the capsular arteries which course around the periphery and the centripetal arteries which penetrate the parenchyma from the mediastinum testis (Fig. 13.10). Flow in the central arteries is directed from the capsule inward (Fig. 13.10). Testicular veins have the same orientation and follow the arteries. Differentiation between arteries and veins affords pulsed Doppler sonography. When the transducer is placed above the testis, the vessels of the testicular cord can be shown (Figs. 13.7d and 13.8b). Under normal conditions only one or two arteries are visible (Fig. 13.7d). It is not possible to show all three arteries simultaneously and to distinguish between the testicular, deferential and cremasteric arteries for sure.


Fig. 13.10
(a–c) Colour (a, b) and power Doppler sonogram (c) of intratesticular vessels of young adolescents. With sensitive flow settings (Nyquist limit of colour flow 1–2 cm/s), intratesticular vessels can be displayed. Some of the vessels are depicted transverse, some longitudinal
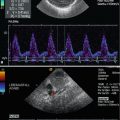
Spectral Doppler shows a low-resistance, monophasic flow pattern within the testis (Fig. 13.11). That means a systolic-diastolic forward flow, with high diastolic amplitude in contrast to the high-resistance flow within the cremasteric artery with low diastolic amplitude. This artery is occasionally visualised along the course of the testicular cord. In adults the peak systolic flow velocities range from 4 to 19 cm/s (mean 9.7 cm/s) and the end-diastolic velocities from 1.6 to 6.9 cm/s (mean 3.6 cm/s) (Middleton et al. 1989).


Fig. 13.11
(a, b) Pulsed Doppler recording of the flow in intratesticular vessels of the testes. The flow profile is characterised by high diastolic amplitude, due to low peripheral resistance
Paltiel and co-workers measured the resistance index of 33 boys from infancy to childhood (Paltiel et al. 1994). They plotted the mean RI against the testicular volume. In testes with a volume under 4 ml, they found a wide range of the RI between 0.39 and 1.00 (mean 0.57). In testes with a volume over 4 ml, the RI ranged from 0.43 to 0.75 (mean 0.57). Mean testicular RI in samples of pubertal and postpubertal boys is decreased compared with the mean RI in prepubertal boys.
In contrast to Paltiel, Schneble found that the resistance index is independent on age and testicular volume with 0.54 ± 0.08 (Schneble et al. 2011). The investigations of Schneble were performed with modern equipment and published in 2010. Paltiel states that diastolic arterial flow is not detectable in normal testes with volumes of 4 ml or less. With high-resolution ultrasound, this statement is no longer true: Schneble could demonstrate low diastolic flow even in small infants (Schneble et al. 2011).
RI values in normal pubertal and postpubertal boys, where testicular volumes exceed 4 ml, are comparable with the values described in adults (Paltiel et al. 1994).
Normal flow velocities in the testicular arteries in childhood were estimated by Schneble and co-workers (Table 13.2) (Schneble et al. 2011).
Table 13.2
Normal values for the flow velocities and resistance indices in dependency of the testicular volume (Schneble et al. 2011)
Testicular volume | 0–1.5 ml | 1.6–2.6 ml | 2.6–6.0 ml | 6.1–12.0 ml | >12 ml |
|---|---|---|---|---|---|
Vs | 1.98 ± 0.62 | 2.78 ± 0.86 | 3.29 ± 0.95 | 4.50 ± 0.88 | 6.22 ± 1.76 |
Ved | 0.85 ± 0.32 | 1.17 ± 0.45 | 1.52 ± 0.69 | 1.98 ± 0.32 | 2.57 ± 0.77 |
RI | 0.54 ± 0.08 | 0.53 ± 0.07 | 0.53 ± 0.08 | 0.54 ± 0.08 | 0.54 ± 0.05 |
In contrast to the investigation of Paltiel, which were performed before 1994, Schneble found with modern equipment in 2010 that the peak systolic flow velocities of the intratesticular arteries do not correlate with the age, but with the volume of the testes (Schneble et al. 2011). He found that there is a nearly linear increase of the flow velocities with increasing testicular volume. The normal values for the flow velocities and resistance indices in dependency of the testicular volume are listed in Table 13.2 (Schneble et al. 2011).
13.6 Indications for Investigation
The main indication for investigation is the differential diagnosis of acute scrotal pain (Kadish and Bolte 1998; Kalfa et al. 2007; Karamazyn et al. 2006; Kass and Lundak 1997; Lam et al. 2005; Siegel 1992). Causes of acute scrotal swelling and pain are spermatic cord torsion, torsion of the testicular appendage, epididymo-orchitis, vasculitis, idiopathic scrotal oedema and strangulated hernia. Additionally colour-coded Doppler sonography can be used in patients with suspected testicular tumours, after testicular trauma and in inflammatory diseases of the scrotum.
In patients with an acute scrotum, three main pathologies have to be differentiated:
Testicular torsion
Epididymo-orchitis
Torsion of the testicular appendage
The most important disease is testicular torsion, as it affords immediate surgical intervention to salvage the testis. Epididymo-orchitis and torsion of the testicular appendage however can be treated conservatively. The combination of high-resolution sonography, colour-coded Doppler sonography and spectral Doppler allows the differentiation of the various diseases (Kadish and Bolte 1998; Kalfa et al. 2007; Karamazyn et al. 2006; Kass and Lundak 1997; Lam et al. 2005; Pepe et al. 2006; Süzer et al. 1997).
13.7 Testicular Torsion
Spermatic cord torsion refers to twisting of the testis or the spermatic cord one or two times in the scrotal sac (Fig. 13.12). The veins are obstructed first causing venous stasis and haemorrhagic infarction, whereas the arteries are blocked afterward comprising blood flow that lead to testicular ischaemia (Günther et al. 2006). Torsion results from abnormal mobility of the testis due to narrow attachment to the scrotal wall.
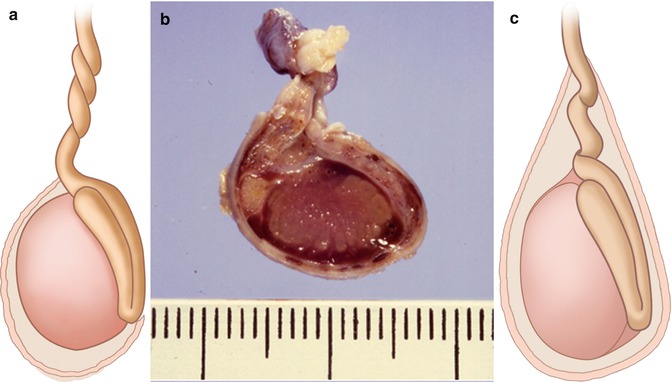

Fig. 13.12
(a, b) Schematic drawing (a) and pathologic-anatomic specimen (Drawing by Blankvisual, Thun, Switzerland) (b) of extravaginal testicular torsion (newborns). (c) Schematic drawing of intravaginal torsion (adolescents) (Drawing by Blankvisual, Thun, Switzerland)
Two types of torsion have to be distinguished: intravaginal and extravaginal (Fig. 13.12). Intra-vaginal torsions are more common (Fig. 13.12c) (Traubici et al. 2003).
Testicular torsion occurs in infants and young adults with two peaks of incidence: the neonatal period and after puberty. In neonates extravaginal torsion (Fig. 13.12a, b) occurs, whereas intravaginal torsion (Fig. 13.12c) is commonly found in later childhood after puberty and in young adults.
13.7.1 Extravaginal Torsion (in Neonatology)
Extravaginal torsion may occur at the level of the spermatic cord, if the cord is poorly fixed in the inguinal canal (Fig.13.12a, b). On the affected side all scrotal contents are strangulated (Traubici et al. 2003). Torsion occurs in utero so that the testis is usually necrotic at birth and is not salvageable by surgery (Fig. 13.12b). Therefore, extravaginal torsion in neonates is not considered as a surgical emergency.
Two-dimensional imaging shows an enlarged heterogeneous testis with hyperechoic and hypoechoic areas. Associated features are hydroceles and scrotal skin thickening (Coley and Siegel 2010). Colour Doppler fails to show flow signals within the affected testis and spermatic cord in contrast to the healthy side. Power Doppler seems to be more sensitive to detect residual flow (Zinn et al. 1998).
13.7.2 Intravaginal Torsion (in Older Children)
Intravaginal torsion occurs when the tunica vaginalis inserts high on the spermatic cord (Fig. 13.12c). The testis is poorly fixed to the scrotum allowing the testis to rotate freely around the spermatic cord (Traubici et al. 2003). The tunica vaginalis completely encircles the epididymis, distal spermatic cord and testis rather than attaching to the posterior aspect of the testis at the site of its mesentery, where it is fixed to the scrotal wall (Dogra et al. 2001). This anomaly is called the bell-clapper deformity because it leaves the testis free to swing and rotate within the tunica vaginalis testis like a clapper inside a bell.
Intravaginal torsion typically occurs in adolescents and young adults. The pathologic process can be divided in acute torsion during which the testis suffers ischaemia but can be saved, if detorsion occurs just in time, and missed torsion, the stage after irreversible ischaemia where the testis can no longer be saved even after detorsion. The testicular salvage rate is about 100 % if surgery is performed within 6 h, 70 % if surgery is performed within 6–12 h and only 20 % if surgery is delayed beyond 12 h after the onset of symptoms (Gronski and Hollman 1998; Patriquin et al. 1993).
Patients usually present with acute onset of scrotal and lower abdominal pain. Associated symptoms are nausea, vomiting, anorexia and low-grade fever.
The affected hemiscrotum is swollen and erythematous. The affected testis is firm and tender and lies transverse and higher in the scrotum in comparison with the contralateral side.
Emergency treatment is necessary to salvage the testis. Immediate operation should not be delayed for unnecessary diagnostic imaging. In unclear cases of acute scrotum however diagnostic imaging makes sense.
Imaging should begin with the presumably normal painless testis for optimal settings of the Doppler device. Intratesticular vessels should be detectable in the normal testis. In a second step the affected testis is investigated. A complete study affords the display of the spermatic cord cranial to the testis (Arce et al. 2002; Baud et al. 1998; Kalfa et al. 2004, 2007; Vijayaraghavan 2006). After two-dimensional imaging of the scrotal contents and the spermatic cord, colour, power and spectral Doppler are performed.
13.7.2.1 Two-Dimensional Image
Greyscale ultrasonographic findings are nonspecific (Dogra et al. 2001). Two-dimensional alterations are heavily dependent on the time between torsion and presentation in the clinic. The involved testis is enlarged in all cases (Figs. 13.13a and 13.14a). This causes an increase in the anterior-posterior diameter. If the difference is 3–4 mm, it is mild; these testes are usually viable (Baud et al. 1998). If the difference exceeds 7 mm, it is marked, which is often associated with a necrotic testis (Baud et al. 1998).



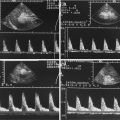
Fig. 13.13
(a) 2D image of testicular torsion of an adolescent boy. Hypoechoic, inhomogeneous echotexture of the enlarged testis. Associated small fluid collections under the tunica albuginea. (b, c) Colour Doppler sonogram of the flow in the left testis with testicular torsion. No flow can be demonstrated in intratesticular vessels of the testis. The peripheral flow in the scrotal wall must not be mistaken as positive perfusion. It is due to anastomoses between the different scrotal arteries. (d) Colour Doppler sonogram of the flow in the normal right testis. In contrast to the torsed testis, flow in the intratesticular vessels can be seen

Fig. 13.14
Testicular torsion in a 12-year-old boy with scrotal pain and scrotal swelling in the last 4 days (Courtesy of Dr. Matthias Röschard, Ravensburg, Germany). (a) Transverse section through the scrotum shows enlargement of the left testis (12 ml) in comparison with the right testis (6 ml). The echogenicity of the left testis is not significantly different from the right testis. (b, c) Colour-coded Doppler sonogram of the boy shows no perfusion of the left testis in comparison with the right one (b). Perfusion of the scrotal wall should not be mistaken as perfusion of the torsed testis. (d) Spectral Doppler shows no flow signals within the affected testis
In testicular torsion the testis lies abnormally transverse in the scrotum. The epididymis cannot be displayed at the cranial pole of the testis (Fig. 13.13a). It may be recognised as a cup-shaped area wrapped around the thickened spermatic cord (Baud et al. 1998). Paratesticular abnormalities are a thickened scrotal wall, an associated hydrocele and an enlarged epididymis due to vascular congestion (Fig. 13.15b).


Fig. 13.15
Mesorchial torsion of the testis in an 18-year-old young man with pain of the scrotum lasting for 1 day. Detorsion of the testis and orchidopexy. The testis was vital at operation and could be saved. Histology revealed partial necrotic tissue (Courtesy of Dr. Gerhard Hammersen, Nürnberg, Germany). (a) Schematic drawing shows that the torsion occurs at the testicular hilus between the testis and the epididymis (Drawing by Blankvisual, Thun, Switzerland). (b) 2D image shows three parenchymal structures within an associated hydrocele: the swollen epididymis (E), the mesorchial torsion (M) and the testis (T). (c) Colour Doppler imaging of the flow within the testis and epididymis. Several vessels are displayed within the epididymis and the area of the torsion characteristic of partial torsion
Alterations of the affected testis are heavily dependent on the time between torsion and presentation in the clinic. They are not a reliable method for the diagnosis of testicular torsion (Arce et al. 2002; Dogra et al. 2001).
In the first hours after torsion, the testis may appear normal in echotexture and size. After 4–6 h the testis enlarges and becomes hypoechoic, due to swelling and oedema (Figs. 13.13a, 13.14a and 13.15b) (Coley and Siegel 2010; Paltiel et al. 1998; Zwiebel et al. 2005). At this time a small hydrocele may be seen (Fig. 13.13a). After 24 h the testis becomes mottled and heterogeneous with hypoechoic areas, due to vascular congestion, haemorrhage and ischaemia (Coley and Siegel 2010; Patriquin et al. 1993; Zerin et al. 1990). Additionally the imaging changes are dependent on the degree of torsion and therefore of ischaemia.
The alterations on greyscale image have been used as a predictor of testicular viability (Dogra et al. 2003; Middleton et al. 1997). Normal echogenicity with mild testicular enlargement is a good sign of viability. Marked testicular enlargement with heterogeneous echogenicity is a bad prognostic feature (Baud et al. 1998).
As greyscale sonographic findings of the testes may be not conclusive, in all patients sonograms of the spermatic cord above the testis and epididymis should be performed. In testicular torsion, the twisted cord produces an abrupt change in the course, size, shape and echotexture of the cord at the point of torsion and below (Fig. 13.16b) (Baud et al. 1998). The twisted cord appears as a round or ovoid mass in an extratesticular location cephalad to the normal inguinal cord (Baud et al. 1998). It is homogeneous or heterogeneous without any specific internal architecture (Baud et al. 1998). The twisted cord has a diameter >10 mm (Baud et al. 1998). When the cord is still vascularised, vascular loops may be seen on the circumference of the cord (Figs. 13.16b and 13.17b). Visualisation of a normal cord course from the inguinal ring to the testis excludes torsion at the time of the examination.


Fig. 13.16
Testicular torsion of the left testis (Courtesy of Prof. Dr. Hirsch, Leipzig, Germany). (a) Transverse section through both testes shows an increased anterior-posterior diameter of the left testis (left image). The echogenicity is normal. (b, c) Spiral twist of the spermatic cord. Located at the upper pole of the testis is a round structure, with a hyperechoic centre and a hypoechoic wall. It is the torsion of the spermatic cord. Colour Doppler clearly shows that the vessels have no straight but a circular course (c), indicative of torsion of the cord
13.7.2.2 Doppler Findings
For the exact diagnosis of testicular torsion, the lack of vascularity in the affected testis in comparison with the non-affected side is typical (Figs. 13.13b, c, d and 13.14b, c) (Waldert et al. 2010).
In complete torsion colour Doppler sonogram shows no intratesticular vessels although a low wall filter and high sensitivity have been used (Figs. 13.13b, c and 13.14b, c).
If no flow can be seen in comparison with the healthy side, immediate surgical intervention is necessary, to salvage the organ. If vessels can be seen, side-to-side comparison of blood flow is essential to exclude partial torsion. The demonstration of intratesticular vessels however does not exclude testicular torsion for sure. Blood flow is cut off completely only with marked levels of torsion (>360°).
In cases of partial torsion intratesticular arteries may only be partially obstructed. Testicular torsion first produces venous obstruction, impaired venous return and venous congestion. This causes swelling of the spermatic cord and testis. In this stage of the disease, arterial perfusion is still present. If the process advances, the artery becomes more and more occluded followed by obstruction of the arterial flow and ultimately testicular ischaemia (Dogra et al. 2001). In these cases additionally spectral Doppler of the flow in intratesticular arteries and imaging of the course of the spermatic cord from the external inguinal ring to the testis have to be performed (Fig. 13.16).
Spectral Doppler shows high-resistance flow in contrast to the monophasic low-resistance flow of the unaffected side (Figs. 13.16f and 13.17c, d) (Dogra et al. 2001; Sanelli et al. 1999). In contrast to normal flow, in testicular torsion the diastolic amplitude is decreased or even absent, whereas the systolic forward flow is still preserved. This causes a marked increase of the resistance index (Figs. 13.16f and 13.17c, d). In severe cases a retrograde diastolic flow in the intratesticular arteries can be found. If the diastolic flow is decreased, emergency surgery is necessary for immediate detorsion and orchidopexy to salvage the testis.


Fig. 13.17
(a) 2D image of spiral twist of the spermatic cord in a 15-year-old boy with acute onset of scrotal pain (4 h). Colour Doppler shows perfusion of the affected testis. Sonography of the spermatic cord above the testis shows an echo-free centre and hypoechoic rim, due to the swollen spermatic cord. (b) Colour Doppler of the flow in the swollen spermatic cord shows spiral twist of the vessels of the spermatic cord characteristic of testicular torsion. (c) Spectral Doppler of the flow in an artery of the partially torsed spermatic cord. Due to partial torsion of the cord, systolic forward flow is preserved, whereas diastolic forward flow is missing. This is caused by high peripheral resistance. Immediate surgery could salvage the testis (a–c) are courtesy of Dr. Jörg Jüngert, Erlangen
Special problems arise on partial or intermittent testicular torsion (ITT). In ITT sonography and Doppler sonography may show inconclusive results. These pitfalls occur because of the indirect evaluation of a condition that is caused elsewhere (evaluation of the testis and not the spermatic cord!) (Arce et al. 2002; Baud et al. 1998; Vijayaraghavan 2006). Therefore, the spermatic cord should be visualised directly, because the torsion occurs there (Fig. 13.16). Arce and Baud suggest to investigate the spermatic cord in its entire length from the inguinal canal to the testis (Arce et al. 2002; Baud et al. 1998). In testicular torsion a spiral twist of the cord from the external inguinal ring to the testis can be found (Fig. 13.16). The thickened cord in the region of the torsion has the appearance of a doughnut, a target, a snail shell or a storm on a weather map (Fig. 13.16b) (Vijayaraghavan 2006). The diameter of the cord often exceeds 10 mm (Baud et al. 1998). If the transducer is moved downward perpendicular to the axis of the mass, a whirlpool sign can be seen in various locations: just distal to the external ring, above the testis or posterior to it (Fig. 13.16c) (Vijayaraghavan 2006). If thickening of the spermatic cord is caused by torsion, often acoustic shadowing can be seen in the region of torsion (Fig. 13.16b, c). It is caused by the twisted various interfaces caused by torsion (Fig. 13.16b, c). Colour Doppler shows flow proximal of the whirlpool and no flow distal to it.
Special problems arise with incomplete torsion. In these patients flow may be seen within the whirlpool mass, distal to it and within the testis (Fig. 13.16e). Incomplete torsion explains previous reports of torsions with preserved testicular perfusion (Bentley et al. 2004). The demonstration of the whirlpool sign helps to overcome this problem. The real-time whirlpool sign is the most definite sign of torsion with 100 % specificity and sensitivity (Fig. 13.16c, e) (Vijayaraghavan 2006).
In incomplete torsion the venous drainage is first compromised, whereas the arterial perfusion is preserved or only partially reduced (diastolic amplitude) (Fig. 13.16f). If the process advances, diastolic flow further decreases until missing or even reverse diastolic flow occurs (Figs. 13.16f and 13.17c, d) (Dogra et al. 2003). Further advancing torsion leads to a decrease of systolic flow until no further flow can be seen (Fig. 13.14d).
Colour Doppler reveals dilated draining veins adjacent to the testis and epididymis.
Another problem arises with intermittent testicular torsion (ITT). In these patients the spermatic cord is straight; the testis and epididymis are swollen and show increased vascularisation. The differentiation from epididymo-orchitis is difficult in individual cases. The differentiation is based on the presence or absence of clinical and laboratory findings. In patients with ITT the testis lies horizontal within the scrotum. Sometimes focal areas of decreased or increased echogenicity can be found within the affected testis. These areas may show a lack of flow with colour Doppler.
The sonographic features of ITT depend on the time between the event and sonographic investigation, the severity of torsion and the duration of the event (Vijayaraghavan 2006).
If the patient reports after a few days of a mild event, the sonographic findings may be normal. If he reports within a few hours after severe torsion and complete detorsion, the testis will be slightly swollen and hypoechoic and the spermatic cord straight. Colour imaging will show hyperaemia (Fig. 13.18c) (Vijayaraghavan 2006). In these cases the differentiation between ITT and inflammation can only be made by laboratory investigation (positive in inflammation) and the horizontal lie of the testis (previous torsion).
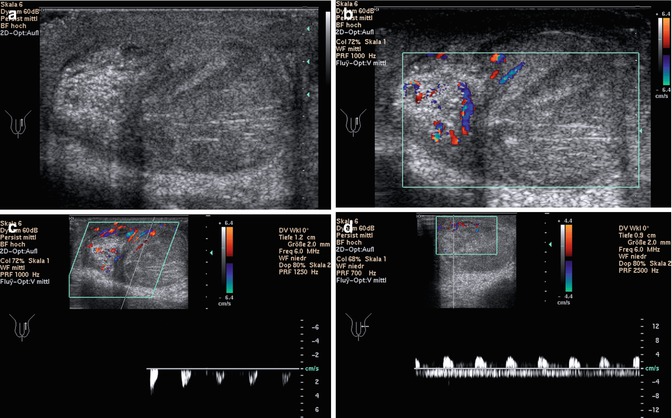

Fig. 13.18
Torsion of the testicular cord. Although the patient was operated immediately, the testis shows haemorrhagic infarction and could not be saved (Courtesy of Dr. Gerhard Hammersen, Nürnberg). (a) Longitudinal section shows increased echogenicity of the testis and especially of the epididymis. (b) Colour Doppler shows vessels within the epididymis, whereas only few vessels are displayed at the cranial pole of the testis. (c, d) Spectral Doppler of the flow in intratesticular arteries. (c) Absent diastolic flow and only low systolic flow can be displayed, characteristic of high peripheral resistance, caused by partial torsion. (d) Pathologic arterial flow above the baseline with absent or even negative diastolic flow, caused by high peripheral resistance. Below the baseline, venous flow is displayed. Due to the venous flow, differentiation between absent and retrograde diastolic arterial flow is not possible
A third group of patients with ITT, who had a severe torsion with complete detorsion, reveal sonographic features of segmental testicular infarction (Kim et al. 2009). The horizontally placed testis shows focal hypoechoic areas without blood flow, whereas the rest of the testis shows normal echogenicity and normal vascularisation (Vijayaraghavan 2006).
13.7.3 Pitfalls of Testicular Torsion
Partial torsion: If torsion does not exceed 360°, blood flow can be seen by colour Doppler although testicular torsion is present. The display of the spermatic cord may show spiral twist of the cord on greyscale image and the whirlpool sign in colour Doppler (Fig. 13.17) (Vijayaraghavan 2006). Another possibility for diagnosis of partial torsion is spectral Doppler, which reveals pathologic high-resistance flow with low diastolic amplitude in intratesticular arteries (Figs. 13.17c and 13.18c, d). This causes an increase of the resistive index. Missing diastolic flow or even retrograde diastolic flow is suspicious of testicular torsion (Figs. 13.17c and 13.18c, d) (Dogra et al. 2001; Sanelli et al. 1999).
Flow within the scrotal capsule: Vessels in the testicular capsule and tunica vaginalis have to be distinguished from intratesticular vessels. Blood flow in these vessels does not exclude testicular torsion. Due to anastomoses between the testicular artery and the cremasteric and deferential artery, flow within the capsular arteries may be preserved even in testicular torsion (Fig. 13.13b).
Reactive hyperaemia of the scrotal wall in missed torsion: In the days after testicular torsion, reactive hyperaemia of the scrotal wall can be seen. In these cases scrotal wall hyperaemia, associated with testicular torsion, may be mistaken for normal intratesticular flow or inflammation.
The torsion of the testis may undergo spontaneous detorsion. The torsion-detorsion phenomenon may show testicular hyperaemia, mimicking an inflammatory process (Middleton et al. 1997; Vijayaraghavan 2006). If the investigation is performed after detorsion in the hyperaemic period, increased blood flow may be mistaken for orchitis or epididymitis (Fig. 13.19c).

Fig. 13.19
Spontaneous detorsion of the spermatic cord (Courtesy of Dr. Jörg Jüngert, Erlangen, Germany). (a, b) 2D image (a) and colour Doppler imaging (b) of the left testis. Normal echogenicity of the affected testis (a). Colour Doppler shows no vessels within the affected left testis on the right side of the image in contrast to normal vascularity in the contralateral healthy testis (transverse section through the scrotum). (c) Colour Doppler after spontaneous detorsion shows reactive increased vascularity in the previously not perfused epididymis and testis
A comparative study between colour Doppler and scintigraphy in patients with testicular torsion showed good correlation of both methods, although there is no gold standard for the diagnosis (Middleton et al. 1990; Nussbaum Blask et al. 2002; Paltiel et al. 1998). A small number of false-negative cases can occur with either modality (Nussbaum Blask et al. 2002). Power Doppler seems to be more sensitive than colour Doppler especially in prepubertal boys (Paltiel et al. 1998; Rubin et al. 1994). In indeterminate cases both modalities may provide complementary information (Nussbaum Blask et al. 2002).
The sensitivity of colour Doppler sonography in the diagnosis of testicular torsion is 90–100 % (Atkinson et al. 1992; Meza et al. 1992; Paltiel et al. 1998; Patriquin et al. 1993). The specificity in technically adequate studies is essentially 100 % (Baker et al. 2000; Burks et al. 1990; Frush and Sheldon 1998; Nussbaum Blask et al. 2002; Weber et al. 2000). If the testicular cord cranial to the testis is investigated in all patients with acute scrotum, the sensitivity and specificity of Duplex sonography increase to 100 % (Vijayaraghavan 2006).
13.8 Torsion of the Appendix Testes and Epididymis
The testicular and epididymal appendages are remnants of the embryonic mesonephric and paramesonephric ducts. The prevalence of appendix testes in the paediatric population is 83.3 %, and that of appendices epididymitis is 20 % (Baldisserotto 2009; Baldisserotto et al. 2005). The appendages testes are usually sessile structures (88 %), whereas the appendices epididymides are commonly stalked (79 %) (Baldisserotto et al. 2005). Stalked appendices predispose them to torsion (Fig. 13.20a, b) (Park et al. 2011). Torsion occurs mainly in prepubertal or pubertal boys. Torsion of the testicular appendages is the most frequent cause of acute scrotum in children (Baldisserotto et al. 2005). The clinical presentation may mimic testicular torsion or epididymo-orchitis.
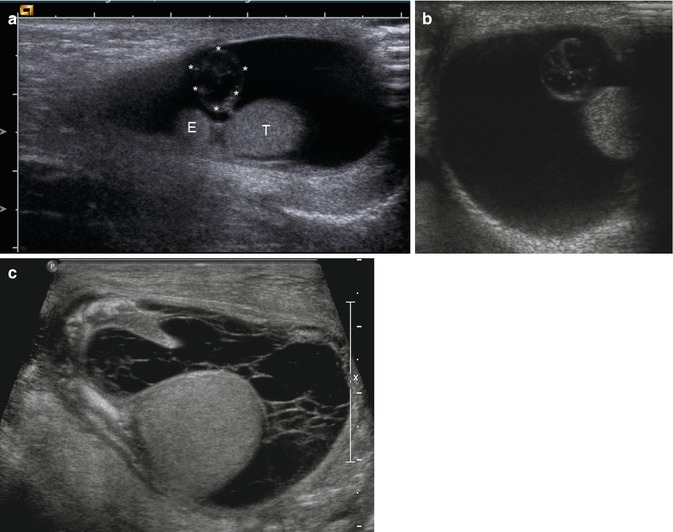

Fig. 13.20
(a, b) Acute torsion of the appendix testis in a 5-week-old boy. (a) longitudinal, (b) transverse section. The image shows a large hydrocele without internal echoes. Beside the normal testis (T) and epididymis (E), a stalked hypoechoic appendix testis (asterisk) with a diameter of 7 mm can be seen. (c) Large hydrocele with internal septa after torsion of the appendix testis (Courtesy of Prof. Helmut Hahn, München, Germany). The fluid, which surrounds the testis, is transversed by multiple fibrin strands
The prevalence of torsion of the testicular appendages varies between 31 and 67 %. The patients present with a tender nodule at the upper pole of the swollen scrotum that may cause bluish colouration, the ‘blue dot sign’.
2D Sonography
The appendix testis is located medially at the groove between the testis and the head of the epididymis. Sonographically normal appendix testes are oval-shaped, isoechoic with the normal epididymis. The appendix epididymis is a long-stalked structure projecting from the head of the epididymis isoechoic with it. In torsion of the appendices, ultrasound shows an extratesticular and extraepididymal mass located at the upper pole of the testis (Fig. 13.20). Torsed appendices increase in size; they have a longitudinal diameter between 4 and 16 mm, usually over 5 mm (Fig. 13.20) (Baldisserotto et al. 2005; Hesser et al. 1993; Park et al. 2011). A testicular appendix larger than 5.6 mm is suspicious of torsion with a specificity of 100 % (Baldisserotto et al. 2005). The echogenicity of a torsed appendix depends on the time from onset of symptoms. It may be hypoechoic, isoechoic and even hyperechoic, sometimes with a hypoechoic centre (Fig. 13.20a, b, 13.21b, 13.22a and 13.23a) (Baldisserotto et al. 2005; Karmazyn et al. 2006; Park et al. 2011). Park et al. could show that all patients with a history <24 h had a hypoechoic appendix testis (Park et al. 2011). The decrease of the echogenicity in the early stage is a result of swelling and congestion. More than 24 h after onset of symptoms, the echogenicity of the nodule varied between hypoechoic, isoechoic and hyperechoic (Park et al. 2011). The increase of the echogenicity is caused by internal haemorrhage and infarction (Park et al. 2011). Normal appendix testes have an echotexture similar to the normal testes (Tables 13.3 and 13.4) (Karmazyn et al. 2006).




Fig. 13.21
Torsion of the appendix testis in an 8-year-old boy with scrotal pain. (a, b) Longitudinal section through the affected testis shows normal echogenicity and size of the testis (T), whereas the echogenicity of the epididymis is increased and the size of the head and tail of the epididymis (E) are enlarged. In the groove between the epididymis and the testis, the torsed echogenic appendix testis (asterisk) can be seen. The spermatic cord (SC) shows a straight course with multiple hypoechoic and hyperechoic parallel lines from the groin to the posterior border of the testis. (c, d) Colour Doppler of the flow in the spermatic cord (c) and the scrotum (d). The images show the parallel course of the vessels of the spermatic cord (SC) and the absent perfusion of the echogenic appendix testis (asterisk) and the normal vascularisation of the epididymis (E) and testis (T). (e) Spectral Doppler of the flow in a testicular artery shows increased diastolic flow caused by vasodilation and reactive hyperperfusion of the testis and epididymis. (f) Intraoperative image shows normal testis and haemorrhagic infarction of the torsed testis

Fig. 13.22
Torsion of the appendix testis in a 10-year-old boy. (a) 2D image shows normal size and echogenicity of the epididymis (E) and testis (T). In the groove between the epididymis and testis, the hyperechogenic torsed appendix testis (asterisk) can be seen. (b) Colour Doppler shows no perfusion of the torsed testicular appendix, whereas the epididymis shows increased perfusion. Testicular perfusion is normal

Fig. 13.23
Torsion of the testicular appendix in a 13-year-old boy with moderate pain in the left scrotum (Courtesy of Dr. Gerhard Hammersen, Nürnberg, Germany). (a, b) 2D image in transverse (a) and longitudinal (b) section through the scrotum. The testis (T) and epididymis (E) have normal size and echogenicity. Adjacent to both structures the oval-shaped swollen torsed appendix testis (asterisks) is displayed within some fluid. (c, d) Colour Doppler imaging shows no perfusion of the appendix testis but good perfusion of the surrounding tissue
Table 13.3
Greyscale sonographic findings of torsion of the testicular cord
Greyscale image |
|---|
Size of testis |
Increased 3–4 mm (mild) |
5–6 mm (moderate) |
≥7 mm (marked) |
Echogenicity of testis |
Homogeneous (<6 h) |
Heterogeneous (>>6 h) |
Associated findings |
Hydrocele |
Scrotal wall thickening (suspicious of missed torsion) |
Spermatic cord |
Abrupt change in course |
Abrupt change in size (>>10 mm) |
Abrupt change in shape (ovoid extratesticular mass) |
Abrupt change in echotexture (homogeneous/heterogeneous) |
Table 13.4
Doppler sonographic findings of torsion of the spermatic cord (sensitive settings—low wall filter, high PRF, appropriate gain). Comparison with the normal testis
Colour or power Doppler sonography of the testis (comparison with contralateral testis) |
No flow in comparison to normal side (complete torsion → OP) |
Reduced flow (partial torsion → spectral Doppler; investigation of the spermatic cord) |
Spectral Doppler of testicular flow (comparison with contralateral testis) |
No flow (complete torsion → OP) |
Reduced diastolic flow and increased resistance index in comparison with the healthy side (partial torsion → OP) |
Colour Doppler of the spermatic cord |
Spiralled vascular loops on cord circumference |
Snail shell appearance of vessels |
Most of the torsed appendices compress the tunica albuginea of the upper pole of the testis (Baldisserotto et al. 2005; Karmazyn et al. 2006).
Associated findings are scrotal wall oedema (55 %) and hydroceles (76–80 %) (Baldisserotto et al. 2005; Karmazyn et al. 2006). The volume of the ipsilateral testis (31 %) and epididymis (73–76 %) is enlarged in the majority of cases, and the echogenicity of the epididymis is increased (Fig. 13.21) (Atkinson et al. 1992; Baldisserotto et al. 2005; Karmazyn et al. 2006).