Fig. 1
A 3-month-old girl with a deaf right ear caused by a congenital cytomegalovirus infection. a Axial postcontrast T1-weighted MR image shows a hyperintense basal turn of the right cochlea (arrow) which can represent contrast enhancement, hemorrhage, or high protein content. No precontrast T1-weighted MR imaging was performed in this patient. b Axial 3D-TSE T2-weighted MR image of the right ear demonstrates no evidence of fibrosis as there is a normal fluid signal in the basal turn with a clear distinction of the scala vestibuli and tympani (arrow). c On the axial multidetector CT (MDCT) image of the right ear, there is no ossification visible in the basal turn, besides a normal dense osseous spiral lamina centrally (arrow). There was a normal cochlear nerve present (not shown), and the patient was successfully treated with a cochlear implant
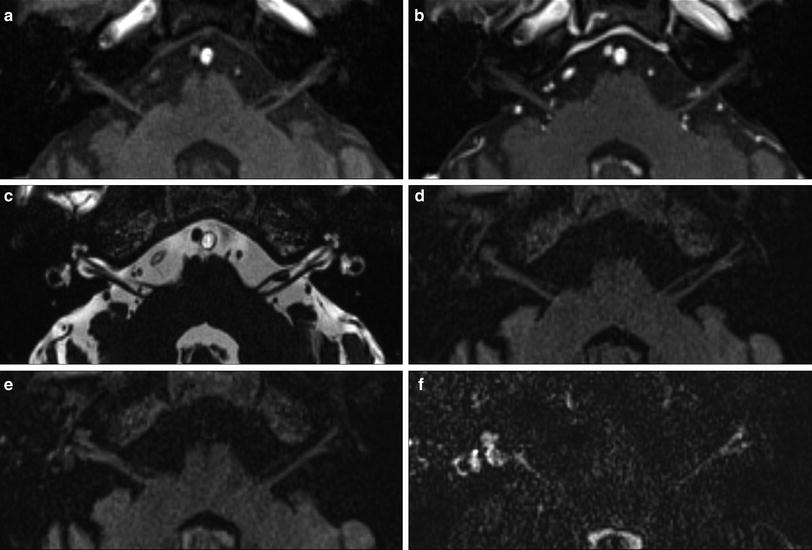
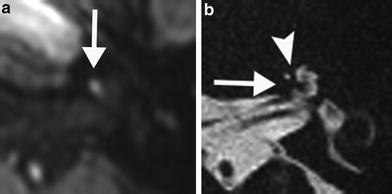
Fig. 2
An 81-year-old man with right-sided facial palsy (Ramsay Hunt syndrome). There were blisters in his right external auditory canal. MR images were obtained 1 week from onset of facial palsy. No significant signal increase of the right labyrinth is noted on the precontrast axial T1-weighted MR image (a) and postcontrast T1-weighted MR image (b). No signal alteration is seen on the axial 3D-CISS MR image (c). Slight signal increase of right labyrinth is visible on the precontrast axial 3D-FLAIR MR image (d), and further signal increase of right labyrinth is visible on the postcontrast axial 3D-FLAIR MR image (e). Remarkable signal increase of right labyrinth is seen on the postcontrast axial heavily T2-weighted 3D-FLAIR MR image (f), showing the extremely high sensitivity of the heavily T2-weighted 3D-FLAIR MR sequence (Courtesy of Shinji Naganawa, MD)
Non-infective etiologies of labyrinthitis are various and include autoimmune diseases such as Cogan’s syndrome, Wegener granulomatosis, relapsing polychondritis, polyarteritis nodosa, systemic lupus erythematosus, Sjögren’s syndrome, ulcerative colitis, and Crohn’s disease (Stone and Francis 2000; Teszler et al. 2008; Benson 2010; Weisert et al. 2012).
Another way to classify labyrinthitis is by cause or origin. Labyrinthitis can be tympanogenic, meningogenic, haematogenic, or traumatic in origin. Tympanogenic spread of infection occurs because of the passage of microorganisms, toxins, or pharmacological agents from the middle ear into the inner ear through the round or oval window (Swartz et al. 1985; Lemmerling et al. 2009). Labyrinthitis can also be due to labyrinthine fistulas which can occur after inflammatory or cholesteatomatous disease (Fig. 3) or postoperatively. Examples of the latter are prosthetic stapedotomy (Fig. 4) and surgery for, e.g., a vestibular schwannoma (Williams and Ayache 2004). Tympanogenic labyrinthitis is typically unilateral. Meningogenic labyrinthitis is usually bilateral (Fig. 5). The source of labyrinthine invasion is thought to be through the cochlear aqueduct or the lamina cribrosa in the vestibule (Hegarty et al. 2002). Bacterial meningitis typically occurs in children and is the most common cause of acquired childhood deafness (Fortnum and Davis 1993). Haematogenic labyrinthitis is the least commonly documented route of spread. Typical causative agents are mumps, measles, human immunodeficiency virus (HIV), cytomegalovirus, syphilis, and borreliosis.
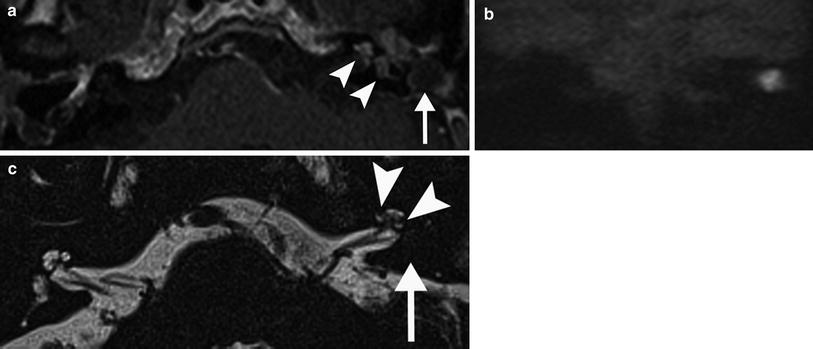
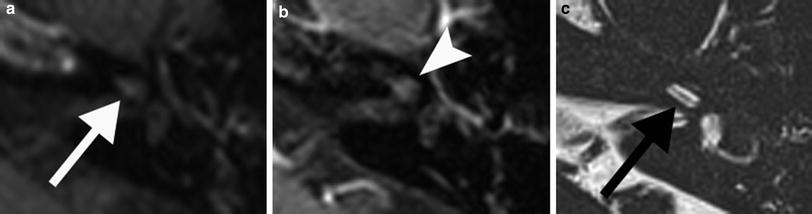
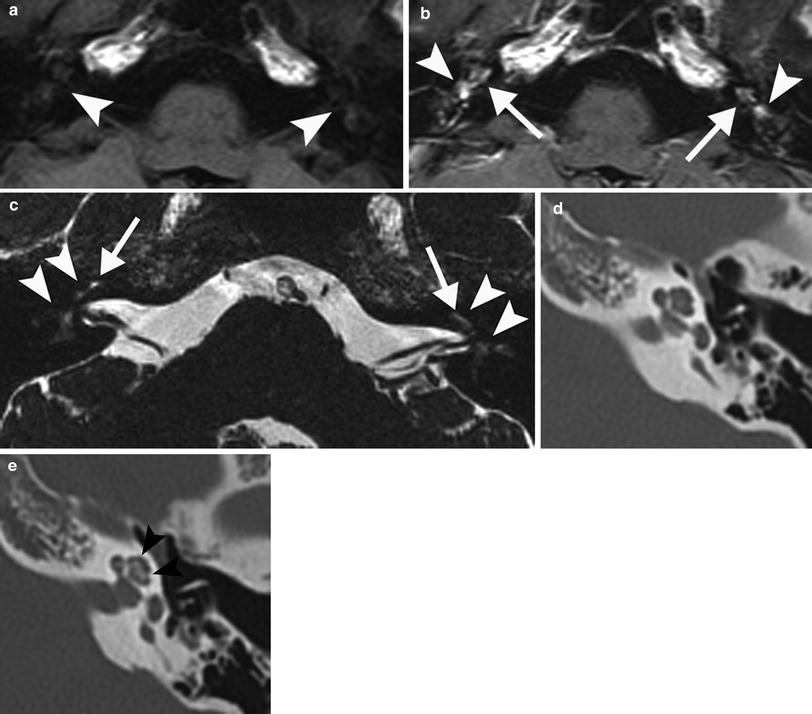

Fig. 3
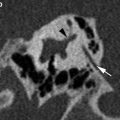
A 61-year-old man patient with left–sided labyrinthitis caused by a cholesteatomatous fistula. a Axial postcontrast T1-weighted MR image demonstrates intense enhancement of the left labyrinth (arrowheads) and the presence of a cholesteatoma (arrow) in the middle ear and mastoid which is clearly diagnosed on the coronal b1000 DWI MR image as a nodular hyperintense lesion (b). c Axial 3D-TSE T2-weighted MR image shows decreased signal in the cochlea with absent signal in the scala vestibuli of the basal and middle turn (arrowheads) and absent signal in the vestibule (arrow). These findings are compatible with combined fibrous and ossified material, which was found during cochlear implantation, performed because the patient was already deaf on the contralateral side

Fig. 4
A 47-year-old man with progressive tinnitus, vertigo, and progressive hearing loss 2 days after stapes surgery for left-sided otosclerosis. a Axial precontrast T1-weighted MR image shows a hyperintense focus (arrow) in the middle turn of the left cochlea compatible with minor hemorrhage or high protein content. b The cochlea enhances further on the postcontrast axial T1-weighted image (arrowhead). c Axial 3D-TSE T2-weighted image demonstrates a normal fluid signal in the middle turn (arrow). The imaging findings are characteristic of acute labyrinthitis

Fig. 5
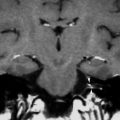
A 45-year-old man with bilateral labyrinthitis and deafness after bacterial meningitis. a Axial precontrast T1-weighted MR image shows no abnormal signal in the labyrinth (arrowheads). b Axial postcontrast T1-weighted MR image demonstrates intense bilateral enhancement of the cochlea (arrows) and vestibule (arrowheads). c Axial CISS T2-weighted MR image shows decreased signal intensity of the labyrinth (arrowheads) with only faint fluid signal remaining in the middle turn of the cochlea (arrows). d Axial MDCT image of the left ear shows no calcification in the cochlea. The imaging findings are consistent with the fibrotic stage of labyrinthitis. e Axial MDCT image of the left ear 4 months later demonstrates extensive ossification of the middle and apical turn (arrowheads) of the cochlea compatible with the chronic stage of labyrinthitis with ossification
2.2 Imaging
Histologically, the evolution of ossification following suppurative labyrinthitis can be classified into three stages: acute, fibrous, and ossification (de Souza et al. 1991; Xu et al. 2009). These stages have also been used in the radiological literature, and they have characteristic features on MRI and CT images (Table 1). In the acute stage, the fluid-filled spaces of the membranous labyrinth (ML) show a normal fluid signal on heavily T2-weighted images. Precontrast T1-weighted images and the more sensitive 3D-FLAIR sequence may show areas of high signal intensity, presumably representing inflammatory exsudate or minor hemorrhage (Casselman et al. 1994; Sugiura et al. 2007). After intravenous administration of gadolinium, T1-weighted images and 3D-FLAIR images demonstrate segmental or diffuse enhancement of the ML due to breakdown of the blood–labyrinth barrier with granulation tissue and angiogenesis (Figs. 1, 2, 3, 4 and 5). CT images will show no abnormality in this stage. When this acute stage of labyrinthitis persists, it progresses to a chronic stage with fibrous changes and later ossification. In the fibrous stage, fibrous strands replace the fluid in the ML, which is seen as a decrease of fluid signal on heavily T2-weighted images (Figs. 3, 5 and 6) (Casselman et al. 1993; Hegarty et al. 2002; Lemmerling et al. 2009; Dubrulle et al. 2010). This stage begins approximately 2 weeks after the onset of infection (de Souza et al. 1991; Xu et al. 2009). There may still be some hyperintense areas visible on pre- and postcontrast T1-weighted and 3D-FLAIR images as the presence of inflammatory exsudate and angiogenesis can persist, but contrast enhancement is less intense than in the acute stage. Differentiation with an intralabyrinthine schwannoma can be made as these tumors will show stronger contrast enhancement and a sharp delineation (Casselman et al. 1993; Dubrulle et al. 2010). CT may show a slight increase in density, but it is still of no use in this stage. The ossification stage is characterized by new bone formation. The evolution to ossification is most commonly a sequela from suppurative labyrinthitis following bacterial meningitis, but other causes are trauma, labyrinthine artery obstruction, autoimmune disease, otosclerosis, leukemia, and temporal bone tumors. In animals, osteoid deposition has been demonstrated as early as 3 days after bacterial infection (Tinling et al. 2004). The fluid-filled spaces of the ML are replaced by this new bone, and there will be no signal visible on heavily T2-weighted images. At this point, usually no abnormality will be seen on T1-weighted and 3D-FLAIR images before or after contrast administration. CT images demonstrate the presence of ossified portions of the labyrinth, and these images are crucial in the precochlear implant evaluation (Figs. 5, 7 and 8). The combination of MRI and CT allows determination of in which scala and to what extent the ossification is present (Fig. 7). Overlap of the three stages can be present (Hegarty et al. 2002; Lemmerling et al. 2009; Xu et al. 2009; Berrettini et al. 2013).
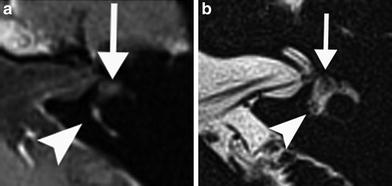
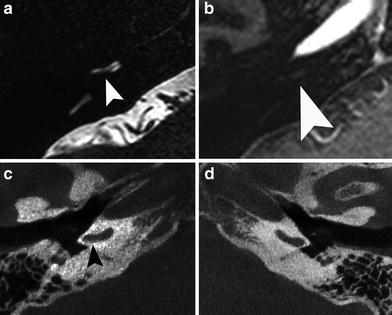
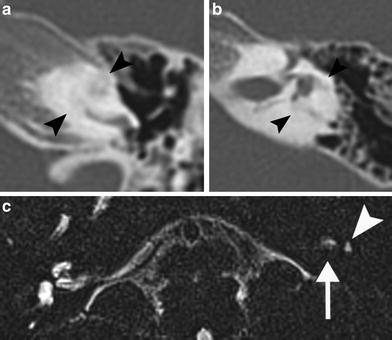
Table 1
MR and CT imaging features in the different stages of labyrinthitis
Stage | T1/3D FLAIR | T2 | T1/3D FLAIR + Gd | CT |
|---|---|---|---|---|
Acute | Normal or intense | Normal | Focal or diffuse enhancement | Normal |
Fibrous | Normal or slightly intense | Decreased | No or slight enhancement | Normal or slightly increased density |
Ossification | Normal | Absent | No enhancement | High density |

Fig. 6
A 23-year-old woman with progressive vertigo on the left since 3 weeks. a Axial postcontrast T1-weighted MR image of the left ear shows slight enhancement of the vestibule (arrowhead) and profound enhancement of the common crus (arrowhead) and posterior semicircular canal. b Axial DRIVE T2-weighted MR image demonstrates decreased signal intensity of the anterior (arrow) and posterior (arrowhead) parts of the vestibule. The imaging features are consistent with the chronic stage of labyrinthitis with fibrosis

Fig. 7
A 45-year-old man who has been deaf bilaterally as a child after meningitis. a Axial DRIVE T2-weighted MR image of the right ear shows an absent signal in the scala tympani (arrowhead) posterior in the basal turn of the right cochlea. b Axial postcontrast T1-weighted MR image shows no enhancement at this level (arrowhead). c Axial cone-beam CT (CBCT) image of the right ear shows a string of ossification (arrowhead) posterior in the basal turn. d Axial CBCT image of the normal contralateral side for comparison. The imaging findings are compatible with the chronic stage of labyrinthitis with ossification

Fig. 8
A 5-year-old girl who is congenitally deaf on the left without a known cause. a Axial MDCT image reveals near-total ossification of the middle, apical and distal basal turn of the left cochlea (arrowheads). b Axial MDCT image shows near-total ossification of the semicircular canals (arrowheads) and part of the vestibule. c No fluid signal is detected in the cochlea and semicircular canals on the axial 3D TSE T2-weighted MR image. Only faint signal is visible in the vestibule (arrowhead) and the fundus of the internal auditory canal (arrow). Extreme example of the chronic stage of labyrinthitis with ossification
2.3 Cogan’s Syndrome
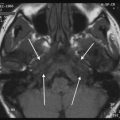
Cogan’s syndrome is a rare systemic disease characterized by non-syphilitic interstitial keratitis and audiovestibular symptoms which are similar to Ménière’s disease (sudden onset of vertigo, tinnitus, nausea, vomiting, and gradual hearing loss). In about 70 % of the patients, there is associated systemic disease considered to be due to vasculitis, usually of the large- and medium-sized vessels. The aetiology and pathogenesis of Cogan’s syndrome are unknown. Initially, it was thought to be a disease caused by infection, with Chlamydia species being the mostly associated culprit. Nowadays, it is believed to be an autoimmune-mediated inflammatory disorder. A prodromen of an upper respiratory tract infection is often reported. The disease mostly affects young Caucasian adults, and there is no sex predilection (Mazlumzadeh and Matteson 2007; Greco et al. 2013). CT and MR imaging of Cogan’s syndrome may be negative, but in most patients, the findings are similar to those of labyrinthitis. CT imaging fails to detect soft tissue changes in the fluid-filled spaces of the membranous labyrinth (ML), but it can detect calcific obliteration in the chronic stage. On MRI, there can be high signal in the ML on unenhanced T1-weighted and 3D-FLAIR images in some patients, which can be attributed to high protein content, but more likely due to hemorrhage as Cogan’s syndrome is associated with vasculitis. Gadolinium enhancement can be seen in the acute stage of the disease and represents gadolinium leakage through the labyrinth membrane. On heavily T2-weighted images, the areas where the fluid-filled spaces of the ML are obliterated show low signal intensity (Fig. 9). The most frequently affected parts of the ML are the semicircular canals, followed by the basal turn of the cochlea and the vestibule (Casselman et al. 1994; Helmchen et al. 1998). Currently, there are no definitive therapeutic recommendations. Intravenous corticosteroids are the first-line agents of treatment, and immunosuppressants are used as second-line drugs (Mazlumzadeh and Matteson 2007; Greco et al. 2013). The hearing loss is generally bilateral and progresses to profound hearing loss in 60 % of cases. In the latter patient group, cochlear implantation remains the only treatment option and has been proven to be beneficial in many cases. In the preoperative evaluation, it is important that the radiologist reports in which scala of the cochlea there is fibrous or calcific obliteration present (Wang et al. 2010; Bovo et al. 2011).
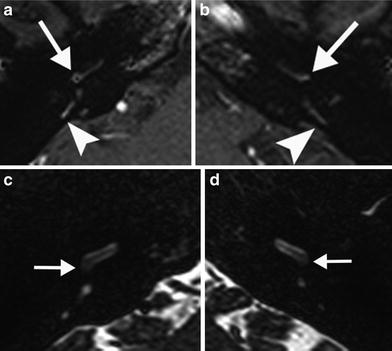

Fig. 9
A 28-year-old woman with recent progressive sensorineural hearing loss caused by Cogan’s syndrome. a, b Axial postcontrast T1-weighted MR image of the right (a) and left (b) temporal bone shows faint bilateral contrast enhancement in the posterior part of the cochlear basal turn (arrows) and posterior semicircular canals (arrowheads). c, d Axial DRIVE T2-weighted MR image of the right (c) and left (d) temporal bone demonstrates a slightly decreased signal in the cochlea bilaterally, most evident in the posterior part of the basal turn (arrows) consistent with a mild degree of fibrosis
2.4 Sudden Sensorineural Hearing Loss
Sudden sensorineural hearing loss (SSNHL) is defined as a sensorineural decrease in hearing of 30 dB or more, affecting at least three consecutive frequencies, occurring over a 72-h period. Up to 90 % of SSNHL is idiopathic at presentation, presumptively attributed to vascular, viral, or multiple etiologies (Stachler et al. 2012). Most MRI examinations performed because of SSNHL will show no inner ear abnormality, and the main purpose of referring patients for an MRI examination is to exclude an underlying tumor (Stokroos et al. 1998). MRI sequences such as 3D-FLAIR are now able to demonstrate labyrinthine fluid changes where the conventional heavily T2-weighted and pre- and postcontrast T1-weighted sequences fail to detect abnormalities (Otake et al. 2006; Tanigawa et al. 2010). In patients with SSNHL, the frequency of high signal found on precontrast 3D-FLAIR ranges from 25.8 to 64.6 % (Cadoni et al. 2006; Yoshida et al. 2008; Ryu et al. 2011; Lee et al. 2012; Berrettini et al. 2013). Of the 13/23 precontrast 3D-FLAIR-positive patients in one study, only three patients demonstrated a hypersignal on a conventional T1-weighted sequence (Berrettini et al. 2013). In two other studies, there was no abnormality visible on T1- and T2-weighted sequences in 31/48 and 12/35 3D-FLAIR-positive patients, respectively (Yoshida et al. 2008; Ryu et al. 2011). High signal intensities in the labyrinth on unenhanced 3D-FLAIR and T1-weighted images may reflect minor hemorrhage or increased protein concentration, and enhancement of the labyrinth and/or the vestibulocochlear nerve after contrast administration is a result of breakdown of the blood–labyrinth barrier (Figs. 10 and 11) (Lee et al. 2012; Berrettini et al. 2013). Most lesions detected on 3D-FLAIR images are isolated to the cochlea, followed by the involvement of the cochlea and the vestibule. Lesions isolated to the vestibule are rare. Vertigo can be present in up to 30 % of patients with SSNHL, and high signal intensities in the vestibule and/or semicircular canals are consistent with the occurrence of vertigo (Ryu et al. 2011; Berrettini et al. 2013). Gadolinium enhancement on 3D-FLAIR images is seen in about half of the cases that are positive on precontrast 3D-FLAIR images (Yoshida et al. 2008; Lee et al. 2012; Berrettini et al. 2013).
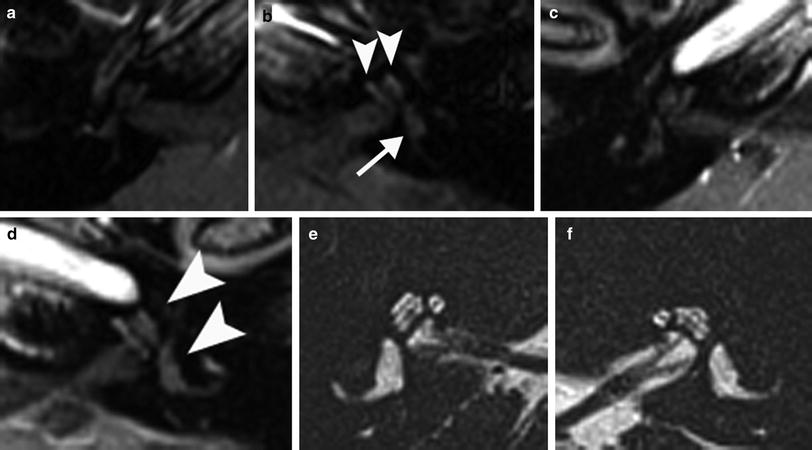
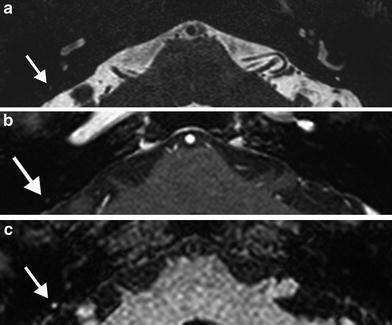

Fig. 10
A 40-year-old woman with left-sided idiopathic sudden sensorineural hearing loss. a, b Axial precontrast T1-weighted MR images of the right (a) and left (b) temporal bone show high signal intensities in the left cochlea (arrowheads in b) and vestibule (arrow in b) reflecting minor hemorrhage or high protein content. c, d Slight further enhancement is seen in the left labyrinth (arrowheads in d) on the postcontrast axial T1-weighted MR image. e, f There is no evidence of fibrotic changes on the axial 3D-TSE T2-weighted MR image

Fig. 11
A 77-year-old woman treated with anticoagulant medicine for cardiac disease presents with sudden sensorineural hearing loss, tinnitus, and vertigo. a Axial DRIVE T2-weighted MR image of the temporal bones demonstrates decreased signal in the posterior semicircular canal on the right (arrow) and slightly increased signal intensity on the postcontrast axial T1-weighted MR image (arrow in b). This high signal is more clearly depicted on the precontrast axial 3D-FLAIR MR image (arrow in c). The clinical diagnosis was compatible with intralabyrinthine hemorrhage. No lesions were detected in the cochlea
3 Intralabyrinthine Hemorrhage
Intralabyrinthine hemorrhage (ILH) is a rare entity. It has been reported in patients with coagulopathy, systemic lupus erythematosus, anemia, sickle cell disease, Cogan’s syndrome, leukemia, trauma, superficial siderosis, cocaine consumption, leukemia, after vestibular schwannoma surgery, and after head and neck irradiation (Casselman et al. 1994; Whitehead et al. 1998; Nicoucar et al. 2005; Sugiura et al. 2006; Poh and Tan 2007; Salomone et al. 2008; Toyama et al. 2009; Dubrulle et al. 2010). SSNHL is the main symptom of ILH, and it can be accompanied by vertigo and tinnitus (Dubrulle et al. 2010). The diagnosis of ILH can be made with MRI where the areas of hemorrhage are spontaneously intense on unenhanced 3D-FLAIR and T1-weighted images (Figs. 11 and 12). These intensities are not well defined, and they do not have a nodular shape which allows differentiation with a lipoma or a schwannoma (Dubrulle et al. 2010). Furthermore, a lipoma will be saturated on fat-suppressed T1-weighted images (Dahlen et al. 2002). An intralabyrinthine schwannoma (ILS) is well defined on postcontrast T1-weighted and heavily T2-weighted images, and it is only slightly intense on unenhanced T1-weighted images compared to the high signal intensity of hemorrhage (Tieleman et al. 2008; De Foer et al. 2009; Lemmerling et al. 2009; Salzman et al. 2012). The high signal areas of ILH on unenhanced T1-weighted images can persist for over a year. After contrast administration, there can be additional diffuse enhancement of the membranous labyrinth. On heavily T2-weighted images, ILH shows a decreased signal of the normal fluid in the membranous labyrinth and these areas are also not well defined nor nodular, allowing differentiation with ILS. This hyposignal suggests the early development of fibrosis which can evolve into ossification (Fig. 13) (Dubrulle et al. 2010). High signal areas on unenhanced T1-weighted images can represent methemoglobin or high lipid content, and the differentiation is usually made based on the underlying condition (Casselman et al. 1994; Whitehead et al. 1998; Dubrulle et al. 2010). SSNHL in general may also be attributable to small amounts of hemorrhage, as discussed in the previous paragraph.
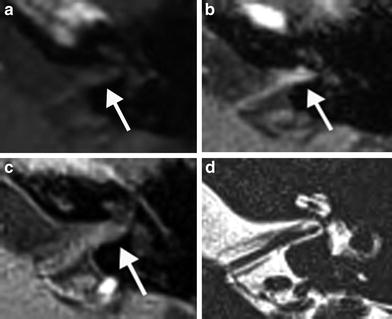
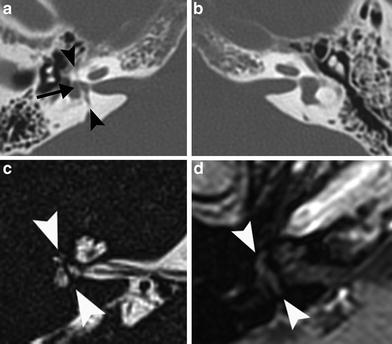

Fig. 12
A 33-year-old man with progressive tinnitus since 2 weeks after commotio cerebri. a Axial precontrast T1-weighted MR image demonstrates a faint hyperintensity (arrow) in the left IAC which is more clearly visible on the axial precontrast 3D-FLAIR MR image (arrow in b). c Axial postcontrast T1-weighted MR image shows subtle enhancement (arrow) of the left IAC. d Normal fluid intensity is present on the axial 3D-TSE T2-weighted MR image. These findings are consistent with posttraumatic hemorrhage in the IAC

Fig. 13
A 49-year-old man with right–sided deafness after skull base trauma at the age of 6, now referred for precochlear implant evaluation. a Axial MDCT image of the right temporal bone shows a transverse fracture line through the vestibule (arrowheads) and a fine string of ossification (arrow) in the vestibule. b Axial MDCT image of the contralateral side for comparison. c Axial 3D-TSE T2-weighted MR image of the right temporal bone shows the absent signal of the string of ossification in the vestibule (arrowheads). There is decreased signal intensity in the vestibule, and no fluid is detected in the semicircular canals consistent with fibrosis. d This abnormal anatomy is also visible on the axial postcontrast T1-weighted MR image (arrowheads), but there is no contrast enhancement
4 Intralabyrinthine Tumor
4.1 Schwannoma
Intralabyrinthine schwannomas (ILS) are benign tumors that spontaneously arise from the perineural Schwann cell sheath of the nerve branches in the cochlea, vestibule, semicircular canals, or a combination of these structures. They initially have no extension to the IAC (Neff et al. 2003). Intracochlear schwannomas develop on the intracochlear branches, and intravestibular schwannomas develop on the vestibular branches which can be the saccular nerve, utricular nerve, and lateral, superior, or posterior ampullary nerve. ILS are rare tumors with less than 200 cases described in the literature to date (Tieleman et al. 2008; Salzman et al. 2012; Bouchetemblé et al. 2013). However, due to the increasing resolution of MRI images, and conspicuity of the radiologist and the clinician, the prevalence of ILS will probably increase. They are reported in association with neurofibromatosis Type 2, but sporadic cases are more prevalent. Hearing loss is the main symptom which is present in practically all patients with ILS. Less common symptoms are tinnitus and vertigo (Tieleman et al. 2008; Salzman et al. 2012). ILS are most frequently located in the cochlea and are often located at the area between the basal and second turn. Most intracochlear schwannomas arise in the scala tympani (Fig. 14), and the remaining intracochlear lesions involve both scalae (Fig. 15). ILS does not appear to be found isolated in the scala vestibuli, and this is thought to be because of the proximity of the nerve to the scala tympani at the habenula perforate, which is the region where the nerve exits the osseous spiral lamina and runs toward the organ of Corti (Fig. 16). The lesions may grow from the scala tympani into the scala vestibuli. The scala tympani ends at the round window and has no connection with the vestibule. Therefore, it is presumed that lesions fill up the cochlea, grow into the scala vestibuli, and consequently grow into the vestibule through the continuous perilymphatic space around the saccule (Figs. 17 and 18). Vice versa, vestibular lesions can grow into the scala vestibuli through this space (Fig. 19) (Tieleman et al. 2008). Tieleman et al. (2008) found most ILS isolated in the vestibule to be in the anterior part with no lesion isolated in the posterior part. Isolated semicircular canal involvement in the non-neurofibromatosis patient seems to be very rare. The same study demonstrated that in only 11.1 % of patients, growth from the cochlea into the IAC could be noticed (Fig. 20). In this largest study on ILS published to date, overall growth rate of ILS was found to be 59.3 %. Serial MRI scan to document growth is therefore the most commonly used management. Hearing preservation is not possible when the ILS is removed, and surgery is considered when there is intractable vertigo and/or tinnitus, or tumor growth extending into the IAC or middle ear (Salzman et al. 2012; Bouchetemblé et al. 2013). Kennedy et al. (2004) proposed the following classification system for ILS: “intravestibular” when ILS is confined to the vestibule with or without involvement of the semicircular canals; “intracochlear” when ILS is confined to the cochlea; “intravestibulocochlear” when ILS is confined to both the cochlea and vestibule; “transmodiolar” when ILS extends from the cochlea through the modiolus into the IAC; “transmacular” when ILS extends from the vestibule through the macula cribrosa into the IAC; “transotic” when ILS extends through the labyrinth into the IAC and middle ear; and “tympanolabyrinthine” when ILS extends through the labyrinth and middle ear.