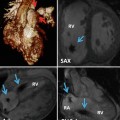
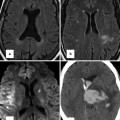
Fig. 15.1
Three-dimensional reproduction of the heart with Ebstein anomaly demonstrating views of the tricuspid valve from short-axis (panel a) and axial imaging (panel b). aRV atrialised right ventricle, fRV functional right ventricle, RVOT right ventricle outflow tract, LA left atrium, LV left ventricle, RA right atrium, TVAL tricuspid valve anterior leaflet, TVPL tricuspid valve posterior leaflet, TVSL tricuspid valve septal leaflet
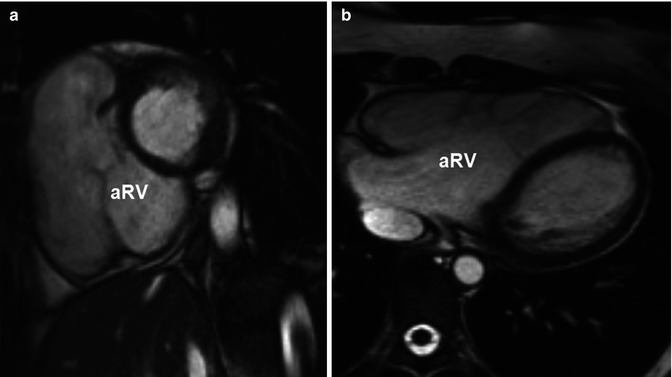
Fig. 15.2
Steady-state free-precession cine images of the atrialised right ventricle (aRV) in the short-axis view (a) and the axial view (b)
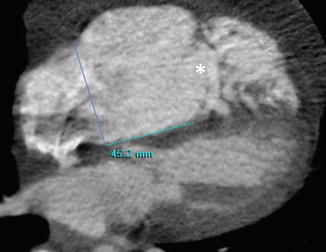
Fig. 15.3
Cardiac CT demonstrates severe dilatation of the right ventricle and apical displacement of the tricuspid valve by 45 mm compared to the mitral septal leaflet insertion. Note the distance between the tricuspid valve annular plane (solid line) and the tricuspid valve coaptation point (asterisk)
Morphology of the Tricuspid Valve Leaflets
At autopsy in 1866, Wilhelm Ebstein described an enlarged and fenestrated anterior leaflet of the tricuspid valve. The posterior and septal leaflets were hypoplastic, thickened and adherent to the right ventricle [6]. Indeed, the leaflets of the tricuspid valve are often thickened and dysplastic in the setting of Ebstein anomaly [7]. Fenestrations, particularly of the anterior leaflet, are often present. In addition, tethering of the anterior leaflet is often seen and is caused by chordal attachments to the ventricular free wall and/or displaced papillary muscles. The anterior leaflet is generally elongated and, if redundant, may obstruct the right ventricular outflow tract [5]. The malformed tricuspid valve leaflets often result in stenosis or regurgitation; however, the latter is far more common. The functional integrity of the tricuspid valve in Ebstein anomaly generally depends on several factors: (a) the degree of displacement of the right atrioventricular junction, (b) the extent of dysplasia and the presence/size of fenestrations, (c) the size and restriction of the anterior leaflet and/or (d) the dimension of the ‘functional’ tricuspid valve annulus. Of note, the degree of displacement of the right atrioventricular junction has important relevance for disease severity and ultimate prognosis in adult patients [8].
Development of the Right Heart Chambers
In addition to tricuspid valve disease, Ebstein anomaly is characterised by a wide spectrum of right ventricular development. In severe forms, the atrialised portion of the right ventricle can become disproportionally large, a finding which has been shown to be associated with poor prognosis in both children [9] and adults [8, 10]. The apex of the functional right ventricle is often heavily trabeculated although the myocardium itself may be thinned or even aneurysmal. In the extreme form, replacement of the myocardium with fibrous tissue has been observed [11]. Although hypoplasia of the functional right ventricle is often seen in neonates and young children with moderate to severe forms of Ebstein anomaly (as a result of downward displacement of the right atrioventricular junction), in adults, conversely, the indexed volume of the functional portion of the right ventricle is typically normal or may even be dilated [12–14]. In two recent studies, dilatation of the functional right ventricle was associated with the severity of tricuspid regurgitation suggesting a mechanistic link between valve insufficiency and enlargement of the functional right ventricle [14, 15] (Fig. 15.4). Unlike neonates, in these studies of adults, there was a linear relationship between the magnitude of apical displacement and the size of the functional right ventricle, likely reflecting an adaptive response to long-standing volume overload from tricuspid regurgitation.
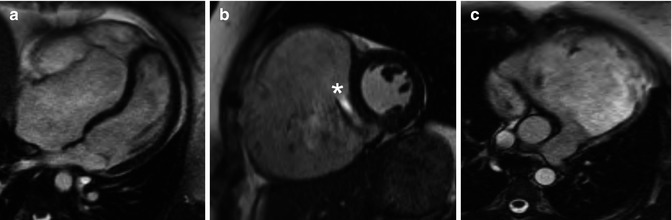

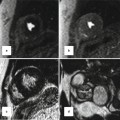
Fig. 15.4
Severe Ebstein anomaly in a 25-year-old woman. These steady-state free-precession cine MRI images demonstrate significant apical displacement of the septal leaflet in the 4-chamber view (a); severe tricuspid regurgitation (* indicates dephasing from a significant regurgitant jet) and severe right ventricular enlargement in the short-axis view (b); and marked dilation of the right ventricular outflow tract in the 4-chamber view (c). The end-diastolic volumes of the ‘functional right ventricle’ was 508 cc (244 cc/m2) in the axial view consistent with severe enlargement (upper limit of normal, 103 cc/m2 for a female)
Associated Cardiac Malformations
Numerous lesions can be seen in conjunction with Ebstein anomaly. Interatrial communications, such as a secundum atrial septal defects or a patent foramen ovale, are the most common and are reported in more than 80 % of cases [16] (Fig. 15.5). Other associated findings include patency of the arterial duct, ventricular septal defects, pulmonary stenosis/atresia, bicuspid aortic valve, aortic stenosis/atresia, coarctation of the aorta and/or mitral valve defects (Fig. 15.6).
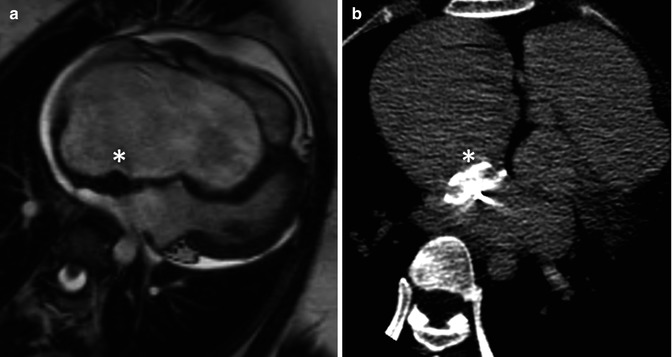
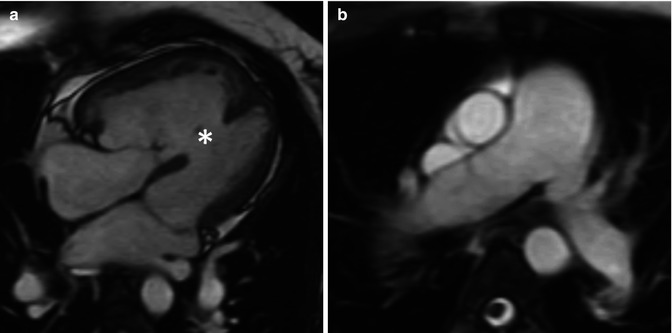

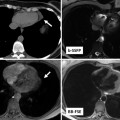
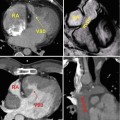
Fig. 15.5
A 32-year-old woman with severe Ebstein anomaly who developed significant cyanosis during pregnancy due to the presence of a patent foramen ovale (PFO). When cyanosis persisted postpartum, she underwent percutaneous device closure of her PFO with an Amplatzer septal occluder, shown here in the 4-chamber view on steady-state free-precession cine MRI imaging (a) and in the axial view on non-contrast CT (b). Note the localised susceptibility artefact related to the device on MRI. After successful deployment of the device, there was full recovery of oxygen saturations

Fig. 15.6
A 22-year-old woman with mild Ebstein anomaly and severe pulmonary hypertension (Eisenmenger physiology) related to a large, unrepaired muscular ventricular septal defect. Based on her systemic pulmonary pressures, her right ventricle is hypertrophied (a) and the pulmonary arteries are enlarged (b). Her prognosis is determined by her pulmonary hypertension rather than her Ebstein anomaly
Congenitally corrected transposition of the great arteries (cc-TGA) is often associated with an Ebstein-like malformation of the tricuspid valve. In this condition, there are discordant atrioventricular and ventriculo-arterial connections. The tricuspid valve is associated with the subaortic ventricle and, therefore, is exposed to higher pressure as compared with typical Ebstein anomaly with concordant atrioventricular connections where the tricuspid valve is associated with the subpulmonic ventricle. The dysplastic tricuspid valve in cc-TGA can occur without or with apical displacement of the septal and posterior leaflets. Unlike classic Ebstein anomaly, in cc-TGA, other typical features tend to be absent (i.e. there is no rotational displacement of the septal and posterior tricuspid leaflets, the anterior leaflet may not be elongated, adherence of the septal and posterior leaflets is limited, the atrialised portion of the right ventricular inflow is generally small, and the inlet portion of the right ventricular myocardium tends not to be dilated or thinned) [17–21].
Of note, left ventricular myocardial abnormalities and left heart lesions are commonly seen in Ebstein disease. In about 20 % of patients with Ebstein anomaly, noncompaction of the left ventricular myocardium has been described [22]. It is important to note that, despite potential links at the time of embryologic development, the extent of noncompaction does not mirror severity of Ebstein anomaly suggesting additional phenotypic modifiers (Fig. 15.7) [22, 23].
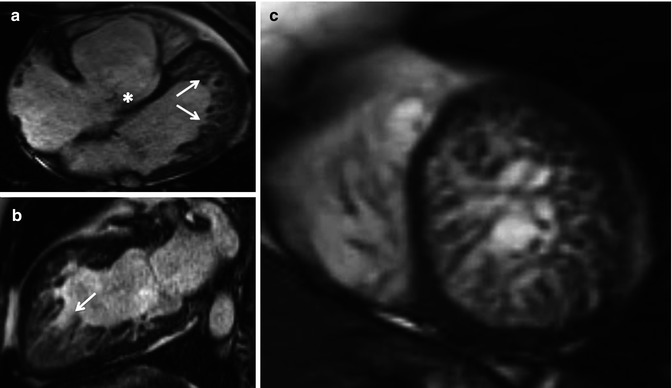

Fig. 15.7
A 40-year-old female with Ebstein anomaly of the tricuspid valve associated with noncompaction of the left ventricle (noncompacted segments predominate at the left ventricular apex and free wall, arrows), on steady-state free-precession cine MR imaging in the 4-chamber view (a), 2-chamber view (b) and short-axis view (c)
The apical displacement of the septal tricuspid valve leaflet may result in discontinuity of the central fibrous body and septal atrioventricular ring, creating a potential substrate for accessory pathway formation. Specifically, Wolff-Parkinson-White syndrome is seen and has been documented in up to 36 % of patients with Ebstein anomaly [24, 25]. In the adult population with further atrial enlargement, patients with Ebstein anomaly are predisposed to other supraventricular arrhythmias such as ectopic atrial tachycardia, atrial flutter or atrial fibrillation [25]. Ventricular arrhythmias (in the setting of myocardial fibrosis or in the context of left ventricular noncompaction) have been described, although are far less common than atrial arrhythmias. [22, 25]
Differential Diagnoses
The most common entities to consider in the differential diagnosis for Ebstein anomaly include tricuspid valve dysplasia and Uhl’s anomaly. Tricuspid valve dysplasia is a condition defined as a spectrum of congenital anomalies of the tricuspid valve leaflets, chordae and papillary muscles often resulting in tricuspid regurgitation. Although the tricuspid valve may be dysplastic or redundant in the setting of Ebstein anomaly (Fig. 15.8), in the case of isolated tricuspid valve dysplasia, there is no apical displacement of the septal leaflet, and ventricular dysplasia is absent (in contrast to Ebstein anomaly). Uhl’s anomaly is characterised by complete or partial absence of the myocardial layer of the right ventricle. Unlike Ebstein anomaly, the tricuspid valve is not involved in the disease process. Uhl’s anomaly is rarely associated with other cardiac malformations.
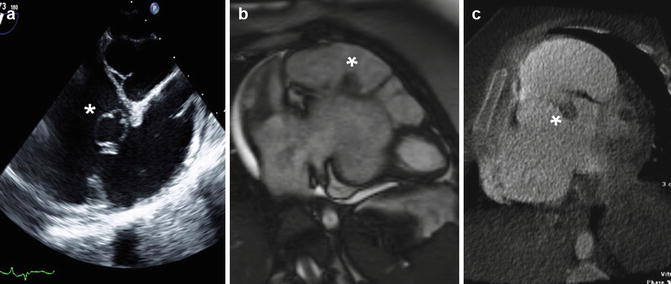

Fig. 15.8
Incidental finding of a cystic structure (1.9 × 1.6 cm) related to the septal leaflet of the tricuspid valve, imaged on transthoracic echocardiography (a), cardiac MRI (b) and cardiac CT (c). Redundant tissue and other abnormalities of the valve leaflets are often seen in the setting of Ebstein anomaly
Clinical Presentation and Treatment Options
In the most extreme form of Ebstein anomaly, cyanosis and heart failure become apparent within the first days of life. Despite recent advances in surgical and medical treatment in congenital heart disease, symptomatic newborns with Ebstein anomaly continue to have dismal outcomes, even in contemporary series [26–28]. Perhaps as a consequence of high mortality in neonatal life, selection bias dictates that adult survivors of Ebstein anomaly will represent the milder form of the disease spectrum.
In milder forms of Ebstein anomaly, patients may be asymptomatic throughout adult life. Although adults may come to medical attention with signs of heart failure or symptomatic arrhythmia [10], fatigue and exercise intolerance are common presentations (Fig. 15.9). Indeed, peak oxygen uptake is reduced in most patients with Ebstein anomaly; the mean aerobic capacity (peak VO2) in a meta-analysis of 230 patients (mean age 22.9 years, 51.5 % male) was about 21 ± 7 ml/min/kg, and half of those studied had an aerobic capacity <60 % of predicted for normal [29]. Decreased aerobic activity has been associated with low resting oxygen saturation [30




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree