• Describe the nature of the electromagnetic spectrum. • Discuss the energy, wavelength, and frequency of each member of the electromagnetic spectrum and how these characteristics affect its behavior in interacting with matter. • Explain the relationship between energy and frequency of electromagnetic radiation. • Explain wave-particle duality as it applies to the electromagnetic spectrum. • Calculate the wavelength or frequency of electromagnetic radiation. • Differentiate between x-rays and gamma rays and the rest of the electromagnetic spectrum. • Identify concepts regarding the electromagnetic spectrum important for the radiographer. • Describe the nature of particulate radiation. • Differentiate between electromagnetic and particulate radiation. In the latter half of the 19th century, the physicist James Maxwell developed his electromagnetic theory, significantly advancing the world of physics. In this theory he explained that all electromagnetic radiation is very similar in that it has no mass, carries energy in waves as electric and magnetic disturbances in space, and travels at the speed of light (Figure 3-1). His work is considered by many to be one of the greatest advances of physics. Electromagnetic radiation may be defined as “an electric and magnetic disturbance traveling through space at the speed of light.” The electromagnetic spectrum is a way of ordering or grouping the different electromagnetic radiations. All of the members of the electromagnetic spectrum have the same velocity (the speed of light or 3 × 108 m/s) and vary only in their energy, wavelength, and frequency. The members of the electromagnetic spectrum from lowest energy to highest are radiowaves, microwaves, infrared light, visible light, ultraviolet light, x-rays, and gamma rays. The wavelengths of the electromagnetic spectrum range from 106 to10-16 meters (m) and the frequencies range from 102 to 1024 hertz (Hz). Wavelength and frequency are discussed shortly. The ranges of energy, frequency, and wavelength of the electromagnetic spectrum are continuous—that is, one constituent blends into the next (Figure 3-2).
Electromagnetic and Particulate Radiation
Electromagnetic Radiation
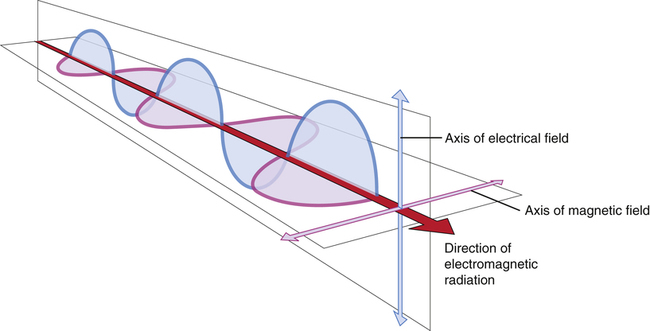
Electromagnetic radiation is energy traveling at the speed of light in waves as an electric and magnetic disturbance in space.
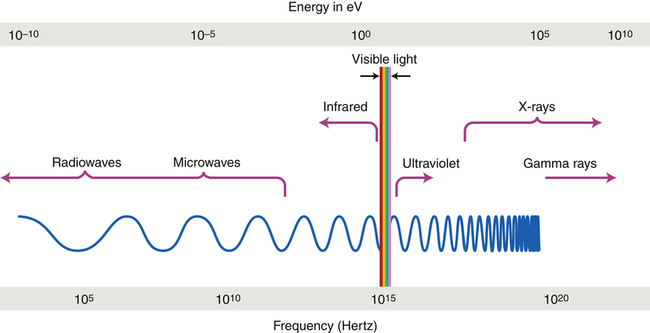
The electromagnetic spectrum energy, frequency, and wavelength ranges are continuous, with energies from 10−12 to 1010 eV.

![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine


