Fig. 2.1
Morphogenesis of the cardiovascular system. (a) Cardiac crescent. (b) Primitive heart tube. (c) Looping. (d) Atrioventricle. (e) Outflow tract. (f) Great vessels. See detail in text. Ao aorta, DA ductus arteriosus, IVC inferior vena cava, LCCA left common carotid artery, LSCA left subclavian artery, MV mitral valve, PA pulmonary arteries, PV pulmonary veins, RCCA right common carotid artery, RSCA right subclavian artery, SVC superior vena cava, TV tricuspid valve
In the cardiac outflow tract, the conotruncal swellings or cushions form the conotruncal septum that divides a tubular structure into two great vessels, namely, the aorta and the pulmonary trunk, in a spiral fashion (Fig. 2.1e). At the bases of great vessels, semilunar valves are formed. A proper connection of the left ventricle to the aorta and the right ventricle to the pulmonary trunk is indispensable for establishment and separation of the systemic and pulmonary circulation after birth.
The great vessel system arises from six pairs of pharyngeal arch arteries which run through pharyngeal arches symmetrically (Fig. 2.1f). The third, fourth, and sixth pharyngeal arch arteries will remodel and transform to a portion of the aortic arch, arch branches, ductus arteriosus, and a part of branch pulmonary arteries.
Developmental Origins of the Heart
Decades of descriptive embryology, including cell lineage tracings have improved our understandings of developmental origins [1–5]. Cells derived from the anterior lateral plate mesoderm form a crescent shape at approximately 2 weeks of gestation, and by 3 weeks of gestation, these cells coalesce along the ventral midline to form a primitive heart tube, consisting of an interior layer of endocardial cells and an exterior layer of myocardial cells, separated by extracellular matrix, or cardiac jelly, for reciprocal signaling between the two layers. The crescent-shaped pool of cardiogenic progenitor cells is now termed “first heart field” (Fig. 2.2a). The first heart field that forms the heart tube eventually contributes to specific chambers of the future heart, exclusively to the left ventricle and all other parts of the heart, except the cardiac outflow tract.
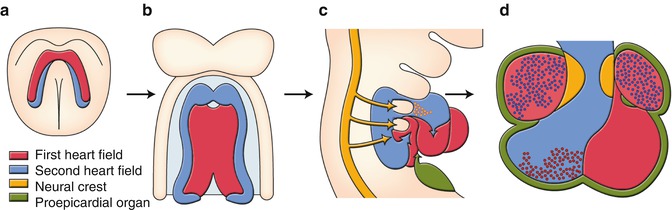

Fig. 2.2
Developmental origins of the heart. (a) Cardiac crescent. (b) Primitive heart tube. (c) Looping heart tube. (d) Four chambered heart. See detail in text
In addition to the first heart field, it was reported 10 years ago that there was a second source of myocardial cells. In mammalian embryos, this source is lying medially to the cardiac crescent (Fig. 2.2a) and then behind the forming heart tube (Fig. 2.2b), extending into the mesodermal layer of the pharyngeal arches (Fig. 2.2c). This second source is termed “second heart field.” The heart tube derived from the first heart field may predominantly provide a scaffold upon which cells from the second heart field migrate into both arterial and venous poles of the heart tube, where they subsequently construct the requisite cardiac components. The contribution of the second heart field to both myocardium and smooth muscle of the arterial pole has been well studied. When the heart tube forms, the second heart field cells migrate into the midline and position themselves dorsal to the heart tube in the pharyngeal mesoderm (Fig. 2.2b). Upon rightward looping of the heart tube, the second heart field cells cross the pharyngeal mesoderm into the anterior and posterior portions, populating a large portion of the outflow tract including future right ventricle and atria (Fig. 2.2c). The addition of the second heart field-derived myocardium to the outflow tract results in its elongation. This elongation is necessary to allow the outflow tract to rotate and shorten sufficiently for correct alignment of the pulmonary and aortic trunks with their respective ventricles.
The third lineage represents cardiac neural crest cells originating from the dorsal neural tube between the mid-otic placode and the caudal boundary of the third somite. After they delaminate from the dorsal neural tube, cardiac neural crest cells migrate into the caudal pharyngeal arches, and the outflow tract where they contribute to the conotruncal cushions that give rise to the outflow tract septum (Fig. 2.2c, d). Migration of the cardiac neural crest cells is also targeted to pharyngeal arches 3, 4, and 6 which give rise to the future great vessels (Fig. 2.2c). Many signaling pathways are involved in the migration and condensation of cardiac neural crest cells, including reciprocal signaling between the cardiac neural crest cells and the second heart field, which are essential for the development of the outflow tract and the aortic arch system.
The fourth lineage of cardiac precursor cells is the proepicardium. The proepicardium develops from the coelomic mesothelium which overlays the liver bud, and the expression of proepicardium-specific genes is induced in naïve mesothelial cells in response to a localized liver-derived signal. Almost all cells of the epicardium and the coronary vessels arise from the proepicardium, which develops as multiple epithelial villi protruding from the pericardial mesothelium immediately posterior to sinoatrium of the looping stage embryonic heart. The proepicardium extends toward the primitive heart and attaches and spreads over the myocardial surface to form the epicardium (Fig. 2.2c, d). During proepicardium growth and epicardial formation, some proepicardial/epicardial cells undergo an epithelial–mesenchymal transformation (EMT) and give rise to the precursors of the coronary vessels and connective tissue cells.
Embryology of the Heart and Congenital Heart Diseases
Congenital heart diseases are considered to occur as a result of the abnormalities of each step in the morphogenesis of the heart and the great vessels. Current embryological concepts for the genesis of congenital heart diseases are discussed below although not all are proved.
Normal and Abnormal Looping of the Heart [6, 7]
The normal left–right development results in situs solitus with all the asymmetric internal organs placed on the body where they should be, and the looping of the primitive heart tube happens to a rightward convex direction (Fig. 2.3b: dextro-loop; d-loop). When left–right specification does not process normally, the direction of looping will be altered and a range of visceroatrial heterotaxia occurs that is usually associated with complex congenital heart diseases. In case of complete mirror-image reversal of all organs, the looping becomes a leftward convex direction (Fig. 2.3a: levo-loop; l-loop), resulting in a benign dextrocardia without significant cardiac malformations and untoward clinical consequences.
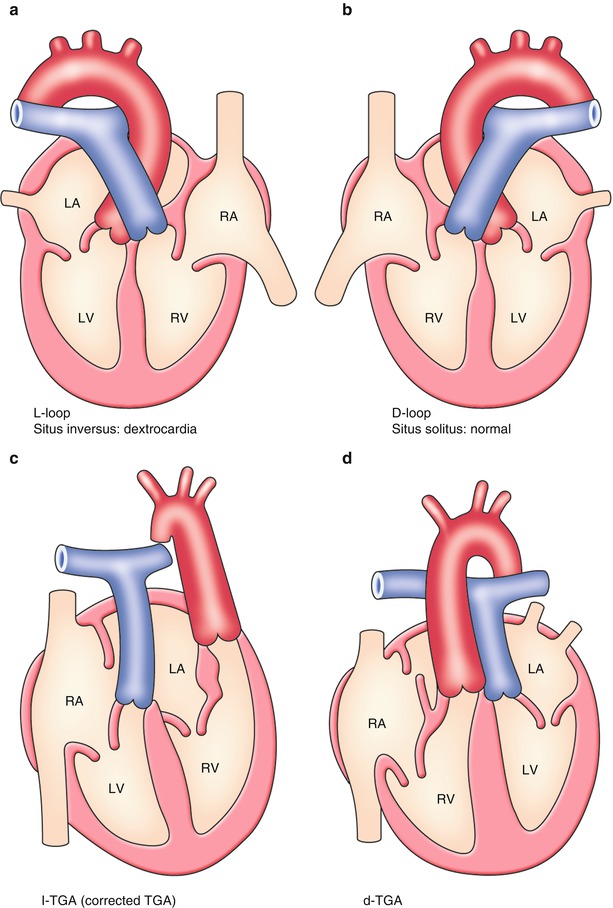

Fig. 2.3
Normal and abnormal looping of the heart. (a) Leftward looping. (b) Rightward looping. (c) L-loop + transposition. (d) D-loop + transposition. See detail in text
Corrected Transposition of Great Arteries (l-TGA)[8]
This phenotype occurs when the primitive heart tube becomes l-loop; at the same time, an arterial trunk is linearly divided into an anteriorly located left-sided aorta and a posteriorly located right-sided pulmonary arterial trunk (Fig. 2.3c). As a result, there is atrioventricular discordance and ventriculoarterial discordance where the right atrium is connected to the left ventricle contiguous to the pulmonary artery and the left atrium is connected to the right ventricle contiguous to the aorta.
Abnormality of Rightward Shift of the Atrioventricular Canal (Inflow Tract): Tricuspid Atresia (TA) [6, 9, 10]
Around 4 weeks of gestation, the heart looping results in a series circulation from the right atrium, to the left atrium, the left ventricle, and the right ventricle that resembles a hemodynamics of tricuspid atresia (Fig. 2.4). Soon later, the atrioventricular canal shifts rightward and the final relation of the right atrium to the right ventricle and the left atrium to the left ventricle is established, resulting in a parallel circulation (Fig. 2.4 lower). The tricuspid atresia is considered to result from an obstacle of the rightward shift of the atrioventricular canal that leads to complete absence of the tricuspid valve with no direct communication between the right atrium and the right ventricle. The atretic tricuspid valve is represented by a dimple in the floor of the right atrium. The resulting membrane is usually muscular, but may be fibrous.
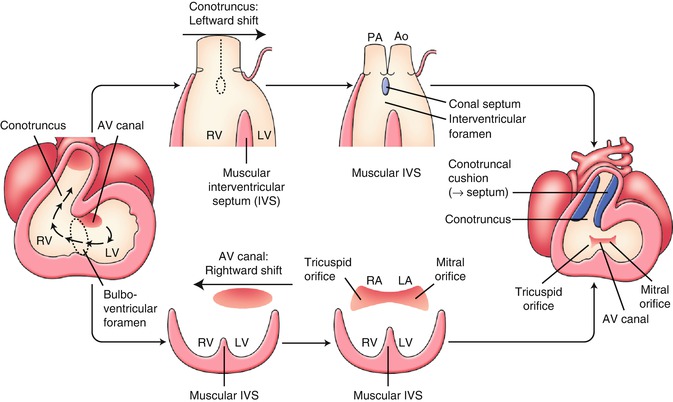

Fig. 2.4
Early development of the inflow tract and the outflow tract. See detail in text. AV atrioventricular, Ao aorta, IVS interventricular septum, LA left atrium, LV left ventricle, PA pulmonary artery, RA right atrium, RV right ventricle
Abnormality of Leftward Shift of the Conotruncus (Outflow Tract): Double Outlet Right Ventricle (DORV) [11–13]
Around 4 weeks of gestation, in a process of the looping, the conotruncus shifts leftward, and the relation of the left ventricle to the aorta and the right ventricle to the pulmonary trunk will be established (Fig. 2.4 upper). DORV, in which both great vessels arise from the right ventricle, is presumed to result from an obstacle to leftward shift of the conotruncus. DORV is also implicated in abnormalities of conotruncal rotation, conus formation, and septal formation. It seems reasonable to accept these abnormalities as a spectrum representing a relatively primitive embryologic condition with the origin of both great arteries from the morphologic right ventricle (Fig. 2.5b). The subaortic conus is normally absorbed during the leftward shift of the conotruncus, and fibrous continuation between mitral and aortic valves can be observed. In DORV, bilateral subarterial conus and discontinuity of both semilunar valves with their respective atrioventricular valve are common.
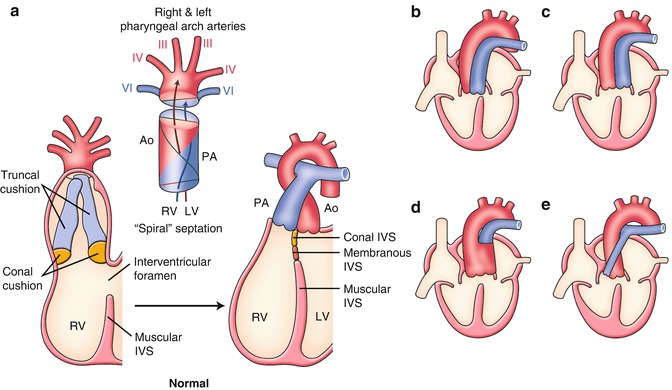

Fig. 2.5
(a) Normal and (b–e) abnormal septation and alignment of the outflow tract. (b) Double outlet right ventricle (DORV). (c) Transposition of the great arteries (TGA). (d) Persistent truncus arteriosus (PTA). (e) Tetralogy of Fallot (TOF). See detail in text. Ao aorta, IVS interventricular septum, LV left ventricle, PA pulmonary artery, RV right ventricle
Normal and Abnormal Septation and Alignment of the Outflow Tract [1, 2, 11–14]
The embryonic outflow tract (conotruncus) consists of a distal part, the truncus, and a proximal part or the conus. At 4–5 weeks of gestation, two big swellings in the truncal region (right superior and left inferior truncal cushions) and two small swellings in the conal region (right dorsal and left ventral conal cushions) develop in the outflow tract (Fig. 2.5a). The coalescence of these four cushions is carried out in a spiral fashion that accounts for the adult gross anatomical relationship between the aorta (to the left ventricle) and the pulmonary arterial trunk (to the right ventricle) (Fig. 2.5a).
There are a variety of clinically important congenital heart diseases associated with abnormalities of the outflow tract. Although they are commonly explained by the developmental abnormalities of cardiac neural crest cells, recently, the involvement of the second heart field for outflow tract defects has been extensively investigated. 22q11.2 deletion syndrome is well known to be highly associated with outflow tract defects.
Transposition of the Great Arteries (TGA) [15]
TGA results from a failure of the outflow tract septation to develop in a spiral fashion, instead it develops in a parallel fashion (Figs. 2.3d and 2.5c). The detailed developmental aspects of the outflow tract septation in a parallel fashion remain largely uncertain. Abnormal development, growth, and absorption of subarterial conus are presumed to be a major factor. Although the normal conus is subpulmonary, left-sided, and anterior, there usually is a subaortic, right-sided, and anterior conus in patients with TGA.
Persistent Truncus Arteriosus (PTA) [1, 2, 12, 14, 16]
PTA results from incomplete formation of the conotruncal septum (Fig. 2.5d




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree