Fig. 7.1
Critical start-up points of the imaging workflow
The imaging core lab should be able to take the lead in generating the imaging charter. The imaging charter may be included as part of a special protocol assessment or scientific advice which will impact the timelines of development and finalization. It will include detailed information regarding image acquisition, read methodology, and data management. The importance of an imaging charter has increased with the draft FDA guidance document released in August 2011, focusing on the content and importance of the imaging charter which is explained in great detail in Chap. 4. The final protocol is imperative to ensure there is no delay in finalizing the imaging charter or the need to produce several amendments.
Imaging manual refers to the detailed instructions regarding image acquisition that is contained within the training manual developed for the investigational sites. This document must fully describe the imaging time points, the imaging modalities, de-identification procedures, image submission procedures, source data storage regulations, query resolution process, and imaging protocol to be followed by the investigational site technologists. The imaging protocol that needs to be followed for a clinical research study differs markedly in comparison to everyday clinical practice. There is much more attention to detail and more documentation involved in performing research studies. Therefore, the technologists need to review the imaging manual document in full before scanning any subjects. We will touch upon this further when we discuss the importance of investigational site training and qualification.
The site survey will capture the investigational sites’ contact information and equipment information which is necessary for ensuring the site is capable of participating in the trial as well as identifying the need for site training when personnel changes occur. Any issues identified with the investigational sites’ equipment capabilities must be flagged to the sponsor and CRO to discuss options and associated risks with that investigational sites participation. In order for the imaging core lab to send the site surveys, they will need to receive a site list from the CRO containing the following required information: investigational site number, investigational site name, study coordinator name, and email address. If this required information is not included in the site list, the imaging core lab will be unable to survey the sites thus possibly causing a delay in start-up. Prioritizing investigational sites for this activity by the study initiation visit dates will be more effective.
Study kits are prepared by the imaging core lab and sent to all the participating investigational sites. A typical study kit will include an imaging binder and media (CDs, films, etc.) and mailers to submit the image data to the imaging core lab. If the imaging core lab has the ability for the investigational sites’ to submit image data electronically and the investigational sites’ have the capability to do so less materials/forms will have to be generated and sent to the sites via courier saving on shipping costs. Just like the site survey, the study kit must be sent to the site at the appropriate time to avoid duplicate work and unnecessary follow-up. This requires clear communication between the imaging core lab and CRO to ensure these activities take place when IRB approval is complete and the SIV is scheduled for the best response from investigational site.
Investigational site qualification refers to the process where the imaging lab certifies that the investigational site is able to successfully conduct all of the procedures required for the clinical trial as detailed in the imaging manual. While this process can increase the time required for having the investigational sites ready to acquire and submit image data, it directly improves the quality of the image data being submitted to the imaging core lab. Qualification can include test scans being submitted for review and approval, phantom scans and instrument quality control. This needs to be highlighted in the risk management plan to ensure the study team takes the appropriate actions with investigational site qualification in respect of time and the imaging modality or modalities involved in the clinical trial. Poor quality scans can have a major effect on the outcome of a trial. Therefore it is imperative that the investigational sites demonstrate proficiency not only at study initiation but throughout the study. This requires ongoing monitoring by the imaging lab.
Investigational Site Training and Qualification
The imaging manual document will need to be generated by the imaging partner. The key elements in maintaining consistency in image acquisition should be highlighted. Investigational sites should identify a primary and a backup technician who will be performing the image acquisitions. Their credentials should be reviewed by the core lab. The technicians should attend the investigator meeting, and special sessions should be devoted to review of the protocol and imaging guideline contents that are relevant. A formal assessment should be performed at the investigator meeting to determine whether the content was understood and is able to be acted upon according to the needs of the trial. Similar to the way the clinical monitor reviews the patient data from the first few subjects in detail with the study investigational sites, the imaging core lab should review the first few images being acquired in detail to ensure that they are consistent with the image standards set up for the trial. Should the image quality not meet prespecified standards, for trials where the imaging assessment is the primary endpoint there is no value in randomizing the subject as without a valid baseline assessment there is no way to generate data on change from baseline. Should there be minor issues with the investigational site these may be managed remotely. However, whenever there are significant issues, trained individuals from the imaging core lab should go to the investigational site, ideally when a patient is scheduled for imaging to assess and remedy the situation. In certain situations such as pivotal phase III trials the sponsor may wish to qualify individual investigational sites prior to permitting randomization of any subjects. This usually involves acquiring images from several patients and sending the images to the core lab for verification of image quality. Once the investigational site has demonstrated proficiency, then they are qualified to begin randomizing subjects.
Investigational site training should not be viewed as a onetime event at the investigator meeting. There will be some imaging technicians who are unable to attend the group training. There will be loss of recall regarding specific procedures over time especially at investigational sites less experienced in conducting these assessments and at slow enrolling investigational sites. A training plan should be requested from the imaging core lab that outlines all activities including investigational site remediation activities that may be required spanning the entire study interval. The training plan should detail how a need for training will be identified proactively via various quality gates established by the imaging core lab.
Study Conduct
Study conduct involves the collection and communication of data between the investigational site, imaging core lab, and study sponsor. The core lab should provide the investigational site with a secure process for transmitting the images together with subject number and core information required for interpreting the images. If the acquired image needs to meet certain criteria for study enrollment, such as a bone mineral density for inclusion in an osteoporosis trial, then turnaround should be sufficiently rapid so as to permit good operational flow at the investigational site level. The same system that securely transmits images between the investigational site and core lab should enable transfer of the images to the blinded readers where assessments can be recorded. Queries pertaining to the data will originate within the core lab to the investigational sites. In the current era of electronic data capture, the imaging core lab should have the ability to do this electronically. The core lab should review with the sponsor the systems, procedures, and data standards that they have in place. They should be able to provide real-time reports regarding the number and type of queries and be able to drill down to the investigational site and subject level upon request. Prior to engaging a core lab, one may want to inquire regarding metrics for similar trials they have performed in the past.
Similar to the conduct of the nonimaging components of the trial, a monitoring plan should be in place for the imaging component. The core lab systems should provide a full audit trail with date and time stamped entries that identify the individual entering the data that are CFR part 11 compliant. In essence the documentation system should allow any auditor to be readily able to reconstruct the events that occurred during the trial. Successfully conducting a clinical trial requires not only technical skills but also good interpersonal communication skills and good attention to detail. As a sponsor one should insist on meeting the team members that the core lab plans to dedicate to your study. You should also inquire as to whether these team members have other significant responsibilities or are dedicated primarily to your project. You should feel comfortable that the team has sufficient experience to solve the problems that will invariably be encountered during the conduct of the trial. Finally, you should agree on a plan for project oversight from both the sponsor and imaging core lab perspective including when certain milestones are achieved such that data will be transferred to the sponsor for assessment of data integrity and analysis.
As we did with study start-up, we are going to now discuss the key tasks and associated best practices for study conduct following the flow diagram in Fig. 7.2.
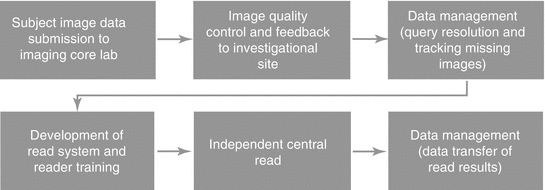

Fig. 7.2
Critical study conduct points of the imaging workflow
The investigational site image data submission to the imaging core lab is an extremely important area that needs to be focused on. Image data must be submitted to the imaging core lab within 3 days of acquisition to maintain high quality. The 3-day window is commonly missed. It is important to understand the process at the investigational sites in order to provide the best solution via electronic submission or simple process improvements at the investigational sites.
If the investigational sites are submitting the image data within 3 days of acquisition, the imaging core lab will have the ability to identify issues early and implement corrective actions with the investigational sites via the image quality control and feedback process. The imaging core lab will need to have highly qualified and certified modality-specific imaging technologists for the required image review per the study protocol and imaging charter. The experience of the imaging technologists is important to ensure imaging-related queries are being generated when required. Some imaging core labs will generate imaging-related queries when they are not necessary or not generate them when they are needed, which will be reflected in the independent central read.
Managing the resolution of queries and tracking down any missing image data from the investigational sites must be ongoing with close collaboration between the CRO and imaging core lab. The process for following up with the investigational sites needs to be clearly stated in the communication plan as well as the appropriate escalation paths when issues need to be escalated. Re-occurring meetings with the imaging core lab and CRO will drive the necessary communication to monitor investigational site, as well as aligning the CRO and the imaging core lab’s activities. Responsiveness from investigational sites is historically poor which is understandable and should be anticipated. Sites commonly do not follow instructions regarding submitting image data as soon as possible after each time point is acquired for each subject. Instead, they send multiple time points together which is referred to as batching. The CRO and imaging core lab must be open in acknowledging these issues and should work together to develop effective solutions.
The development of the read system and reader training has to be completed in order for the central read to commence. There are numerous tasks that have to occur for these activities to be completed. Therefore, the imaging core lab must set the appropriate expectations, roles, and responsibilities to ensure each task is completed and nothing gets overlooked. If one of these tasks is overlooked, it may very well impact the ability to deliver the read results when required. Flexibility in designing a read system can easily improve the power of your data by being able to perform additional analysis. Experienced imaging core labs will bring this to your attention and involve the relevant experts when necessary. Reader training is best accomplished in a face-to-face meeting with imaging core lab, readers (radiologists, oncologists, cardiologists, etc.), sponsor and/or CRO. The ability of the imaging core lab to calibrate the readers through the initial reader training will be reflected in the adjudication rate. Adjudication rates vary per indication, and an experienced imaging core lab will be able to advise you on what is expected and what is abnormal before the independent central read begins.
Once the independent central read has begun, the imaging core lab is responsible for communicating status updates and loading all available subjects into the read systems. The rule for when a subject is to be read needs to be established well in advance. The imaging core lab will use the read plan in order to monitor expected vs. actuals. A bell-shaped enrollment curve is desired by the imaging core lab as it prevents the need to shorten timelines or add resources for the interim and/or final data analysis like when there is a bolus of subjects enrolled at the end of the enrollment period. The time from final read to interim or final analysis needs to be looked at closely as the time necessary to complete this task will vary per read methodology, the required image data being available to be read, the selected readers’ availability, and time needed to send the data transfer containing the read results.
Data transfer of read results should be a smooth process as it is the final critical point in the process when tension is at its highest point. An experienced imaging core lab will start discussions of the data transfer early and finalize the required specification document shortly after the design of the read system has been finalized. The sooner this can be done and a test transfer can be generated and approved by the recipient (sponsor, CRO, or third party), the better as it allows flexibility to review the read results earlier than expected if necessary.
Once the final data transfer is completed many imaging core labs feel that their work is done for the most part which is incorrect. An experienced imaging core lab will assist with the interpretation of the data from the images with the health authority submission. Presentation can make or break any deal in real life and this also rings true with submission. You have to put the results in context of both a clinical and therapeutic response. An imaging core lab advising the sponsor about the data significance will be a great asset to the health authority submission.
Risk Mitigation Plan
In addition to the monitoring plan, a risk mitigation plan for the imaging component is another document that will benefit the clinical trial immensely. The imaging partner should lead this process by going through deviations from the intended imaging process and should gain consensus on how to manage these deviations prior to study initiation. This should all be clearly detailed in a risk management document that focuses on the foreseeable risks specific to the clinical trial. Risks associated with investigational site start-up, investigational site training, missing images, resolution of queries, independent review progress, data transfers are all crucial to discuss at the start of the clinical trial, and this open dialog needs to continue throughout the trial. Transparency between all parties is critical to success. The experience of an imaging core lab feeds into this document and is a good test to determine if you selected an experienced imaging core lab or not.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree