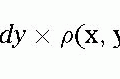Extra Thoracic Magnetic Resonance Angiography
Robert W. W. Biederman
Generally, in discussions of cardiovascular magnetic resonance (CMR) most authors limit their discussions to supradiaphragmatic anatomic and pathologic vascular perturbations. We, however, believe that nonthoracic aortic vasculature should also be included. The purpose of this chapter is to emphasize to the clinician that the “C” in CMR stands for cardiovascular, and therefore includes the broader vasculature and is not limited to that adjacent to, or associated with, the small beating muscle in the center of the chest. The following will be a general discussion on the utility of CMR, with and without the use of magnetic resonance angiography (MRA), to understand peripheral angiography. Over the last decade, extensive research and investigations have demonstrated the utility, applicability, and reproducibility of noninvasive imaging of the peripheral vasculature using CMR. Many specialized textbooks are dedicated to that subject alone, and this single chapter will serve to summarize the capabilities of CMR. Specifically, it is not meant to be an exhaustive compendium of all that is known. In this chapter we will not focus on specific aortic disease as an individual entity, but only as related to conduits of the branches derived from the aorta with the exception of the carotid vessels, which are not included.
Many noninvasive assessments of peripheral vascular disease exist, including ultrasound which incorporates continuous wave, pulse wave Doppler, and two-dimensional imaging; exercise testing; impedance plethsmography; and magnetic resonance imaging (MRI)/MRA. Each of these noninvasive modalities has its own advantages and disadvantages. Before the development of MRA, the only suitable method to evaluate the peripheral vasculature was contrast arteriography. Now, virtually all vascular territories can be visualized by the use of, either in combination or individually, CMR and MRA. Although this chapter will be limited to arterial structure it should be noted that there is also a role for venous and lymphatic characterization by both anatomic and flow-related techniques.
As the population continues to age, the role atherosclerosis plays cannot be overstated. Beyond affecting the coronary bed, the aorta and the carotids, there is increasing recognition that impairment of the renal arteries and peripheral runoff vessels are major sources of morbidity and mortality. Unexplained hypertension affects a significant number of patients because of occlusion of one or more renal arteries. Claudication has been recently recognized by the National Institutes of Health (NIH) as one of the major morbidities facing our aging population, and strategies aimed at detecting its presence are in general under utilized by the internist, cardiologist, and the general surgeon. Historically, a major difficulty in this assessment was a lack of a suitable imaging strategy, placing the vascular surgeon, cardiologist, and interventionalist at a disadvantage when addressing this major disabling entity. With the recognition that diabetes plays a growing role in disease presentation, either due to its increase prevalence or better recognition, the search for occult atherosclerotic disease in this population has been recognized as being critically important by leading medical societies. Peripheral arterial disease is the most common cause of systematic obstruction in the major extracoronary arteries. For the most part, the pathophysiologic understanding of this disease process is identical to that of the coronary artery tree. The risk factors for atherosclerosis of the noncoronary arteries are identical to those of coronary artery disease patients. Detection by CMR follows a similar pathway to that utilized in invasive strategies, aiming to detect pathology that exceeds 50% obstruction. In general, as the disease process progresses, luminal encroachment graded as >70% is likely to be responsible for symptomotology. Claudication, whether it is of the lower extremity or upper extremity through effort related activity, is almost always the universal presenting symptom. Intermittent claudication can be mild, progressive, and debilitating and can wax and wane.
Because the therapies for peripheral arterial disease, while still in their infancy, have generally improved over the last decade, the CMR capability to detect disease has progressed rapidly. Despite these improved imaging strategies, detection of the disease does not always lead to optimized therapy for all vascular territories. For instance, the external iliac arteries, a source of substantial debilitating buttocks claudication, are rarely intervened upon. A chief therapeutic option remains adopting conservative measures, strongly focusing on preventing progression of disease, improving exercise capability with walking, conditioning exercises, and cessation of smoking. Several pharmocologic therapies are available but have not generally resulted in dramatic improvements in symptoms. The fall-back position for patients who have failed conservative therapy is interventional vascular or surgical therapies, which have shown great promise in reducing certain morbidities and mortalities. The characteristics of CMR are ideal in detecting atherosclerosis within the peripheral vascular bed; early experimental research suggesting this may be an important modality to risk-stratify atherosclerotic plaques.
SPECIFIC CARDIAC MAGNETIC RESONANCE IMAGING TECHNIQUES
Utilizing both the double inversion recovery (DIR) and the steady state free precession (SSFP) sequence, the great majority of aortic iliac disease can be diagnosed. The SSFP sequence is an improvement over the older, flow-dependent, gradient recalled echo (GRE) sequence in that turbulent flow, which previously resulted in overestimation of disease state, is less problematic. Both axial and coronal images are ideal for this presentation. Utilizing a combination of SSFP and localized axial sequences to grade disease state more effectively, the territory of the ileofemoral and infrageniculate arteries can be defined. Compared to dynamic imaging, the addition of gadolinium-based MRA sequences offer substantial improvement in visualization, which leads to increased confidence in diagnostic interpretations. Therefore, in our laboratory all peripheral vascular studies, despite the level of confidence obtained with noncontrast imaging, are completed with MRA. Only in the rare instance in which the patient cannot undergo intravenous administration, will we attempt to utilize time of flight (TOF) imaging. This contrasts with examination of the aorta in which non-contrast imaging is often sufficient to not require MRA.
With three-dimensional (3-D) MRA, surface and volume rendering views are obtained, providing high-quality information rapidly (see Fig. 11-1). However, on occasion, reconstruction information is equivocal. In these cases, returning to the source images and scrolling through them serially is recommended. Interrogating the axial, sagittal, and coronal images as source data permits more accurate interpretation, because the data are not summated. The T1-weighted sequences with blood suppression produce images in which turbulent flow from highly stenotic jets is suppressed, and is therefore not a source of error in overestimation of the degree of stenosis. Importantly, the MRA reconstructions can be used as a source of “vascular roadmaps” for the interventionalist and for the surgeon. This is in addition to simple screening for aiding in decision making regarding interventional strategies (see Fig. 11-2). Perhaps the cardinal advantage of MRA is the ability to define arterial collateralization in a meaningful manner. Several studies have demonstrated the increased ability of MRA compared with angiography to detect collaterals that can be utilized in revascularization strategies (see Fig. 11-3). Additionally, studies have documented the ability of CMR to be performed in a painless and rapid manner as compared with x-ray angiography. The most rapidly growing section of CMR is in the area of peripheral angiographic evaluation (see Fig. 11-4), specifically for runoff vessels.
CASE STUDIES
Case Study 1.
A 65-year-old man presents with left leg claudication, and on examination a left proximal iliac bruit is thought to be heard (see Fig. 11-2, top left panel). The patient is referred for CMR ileofemoral angiography. The distal abdominal aorta is well visualized, as is the right ileofemoral system. Arising at the ostial left iliac artery is a long 2.5-cm lesion with clean “cutoff” sign. A very faint “string sign” is also seen. The combination is indicative of a subtotal occlusion. The referring physician was notified and the patient was promptly sent for x-ray angiography with a plan for a left iliac angioplasty/stent. The following day I received a call from the angiographer stating, “There are widely patent bilateral iliac arteries. The site of the proximal right iliac stent is also widely patent!” Unbeknownst to all, the patient had earlier undergone implantation of one of the first iliac stents to be placed in the state. This is now a well-known artifact. It requires vigilance on the part of the interpreter, who may not necessarily be privy to all of the necessary information. The clean cutoff sign is now retrospectively, a clear indication of a nonanatomic stenosis associated with a stent paramagnetic artifact and due to the “shielding” effect of the stent.
Case Study 2.
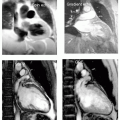
A 67-year-old man status post bilateral femoral artery conduits now with two symmetrical peculiar bilateral aneurysmal formations at the anastamosis to the native vessel, imaged with coronal SSFP (see Fig. 11-2, lower left panel) and confirmed by MRA (see Fig. 11-2, lower right panel). Note the prior aneurysm clips seen as paramagnetic artifact appearing to deform the bladder (filling with the T1-weighted contrast), Fig. 11-2, lower left panel. An additional SSFP (see Fig. 11-2, upper right panel) reveals a large mass in the abdomen that is a partially undigested heavy breakfast masquerading as a large mass.
Case Study 3.
A 65-year-old white man presents with right leg and buttocks pain and claudication. An x-ray angiogram was not definitive in explaining the nature of the vascular problem despite digital subtraction angiography (DSA), mostly due to what appeared as a plethora of collaterals (see Fig. 11-3). A single-station runoff is performed which clearly demonstrates substantial epigastric to ileofemoral collaterals as well as external iliac stenosis. The patient underwent iliac to femoral bypass with improvement and reduction, but not complete elimination, of the right buttocks pain (presumably due to retrograde flow to the external iliac arteries).
Case Study 4.
A 22-second breath-hold “stem-to-stern” MRA was performed in a 45-year-old man (see Fig. 11-4). One single large FOV acquisition was acquired, delineating nearly the entire thoracic aorta (only the cranial arch is not seen) extending throughout the descending thoracic aorta, entire abdominal aorta, and proximal ileofemoral vasculature. Note the ostial and proximal/mid superior mesenteric artery (SMA), inferior mesenteric artery (IMA), and renal arteries. Three-dimensional surface reconstruction was reformatted and selected images were displayed. By altering the signal intensity of the post-processing algorithm, increased or decreased visualization of the vasculature can be achieved.
Case Study 5.
A 60-year-old woman status post coronary artery bypass graft (CABG) 5 years now reports progressive chest discomfort, radiating deep into her right chest with a ripping, tearing sensation completely different from her initial chest pain symptoms that resulted in bypass surgery. The CMR demonstrated a complex, spiral dissection originating from the origin of, but not directly involving, the left subclavian artery, extending throughout the entire thoracic aorta and deep into the abdominal aorta (see Fig. 11-5). A number of auto-fenestrations are visible (arrow), as well as evidence that the left renal artery is supplied by the true lumen (arrow). On other images (not shown), the right renal flow is seen to be derived from the false lumen. However, in that the flow characteristics of the descending aorta revealed nearly simultaneous signal intensity, it is reasonable to assume that flow is nearly simultaneous. The generally slightly brighter signal in the false lumen (right) as compared to the true lumen (left) denotes the slightly faster blood flow, likely not physiologically significant.
Case Study 6.
Surface rendered MRA of a 9-second scan performed in a 51-year-old man presenting with chest and abdominal pain (see Fig. 11-6). In the upper right panel, a very subtle defect is seen, inferior to the ostium of the left renal artery. Using both DIR and SSFP imaging, in combination with the volume rendered MRA, no evidence of dissection is seen, although suspected in the SSFP sequence. Incorporating both contrast and noncontrast imaging demonstrates this is an atherosclerotic plaque, but not the likely source of the patient’s pain. Note, also from the surface rendering angiogram in the middle panel that anatomic and arterial information from the right and left ventricle, as well as the mesenteric, renal, and ileofemoral system are well displayed. Even the vasculature supply to the small and large bowels is reasonably well depicted as is the right ureter. Note the high-fidelity resolution of the MRA reformatting possible with (left panel) and without (middle panel) the small vessel vasculature all dependant on the intensity leveling reformat chosen.
Case Study 7.
An abdominal aorta, mesenteric and renal artery MRA acquired as a staged 10-second bolus-tracking acquisition, demonstrating in fine detail the SMA, IMA, and renal arteries extending to the secondary and tertiary branches (see Fig. 11-7). The common iliac system is also visualized in this 40-year-old man referred for hypertension, and shown to have no evidence of renal artery stenosis.
Case Study 8.
MRA of the renal artery with bilateral accessory arteries obtained in a 52-year-old woman referred for systemic hypertension (see Fig. 11-8). No evidence of renal artery stenosis is present. The MRA allows visualization of the vasculature deep into the renal cortical medullary territory. A renal cyst is also present as depicted by the lack of contrast uptake.