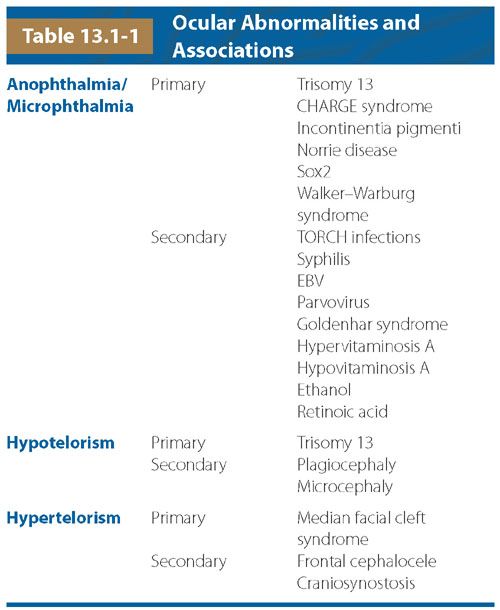
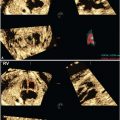
FIGURE 13.1-1: Orbital Measurements by US. Transorbital view of the fetal face shows the measurement of the BOD with the calipers on the malar margins of the orbit. The IOD is measured between the two ethmoidal margins (arrows).
On T2-weighted MRI, the whole lens is low signal compared with the high signal of the vitreous. The BOD and IOD measurements can be made in any plane from true axial to true coronal, provided both eyes can be seen in the same image and have the most equal and largest possible transverse diameters. The binocular and interocular distances are, respectively, measured between the two malar or ethmoidal margins of each vitreous (Fig. 13.1-2). These measurements can be plotted against gestational age (see Table 26 in Appendix A1).2 Other available nomograms additionally include measurements of the transverse diameter of the lens9 and anteroposterior diameter of the globe.10 It has been noted that the globe is ellipsoid earlier in gestation. This is thought to be attributable to the presence of the hyaloid artery initially tethering the lens to the optic disc. With involution of the hyaloid artery, the globe is increased in anteroposterior diameter.

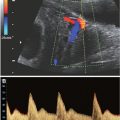
FIGURE 13.1-2: Orbital Measurements by MRI. A: The BOD is measured between the two malar margins of each high-signal vitreous. The measurements can be made in any plane orthogonal to the sagittal plane. B: Here, the IOD is measured in the coronal plane between the two orbital margins of each high-signal vitreous. (Reproduced from Robinson AJ, Blaser S, Toi A, et al. Magnetic resonance imaging of the fetal eyes: morphologic and biometric assessment for abnormal development with ultrasonographic and clinicopathologic correlation. Pediatr Radiol. 2008;38:971–981, with permission of Springer-Verlag.)
ANOPHTHALMIA/MICROPHTHALMIA
Anophthalmia and microphthalmia can really only be differentiated pathologically. Anophthalmia is complete absence of the globe but in the presence of the ocular adnexa (eyelids, conjunctiva, and lacrimal apparatus). Microphthalmia by definition means small globe. It should be differentiated from cyptophthalmos, that is a failure of separation of the eyelids, which should occur by 24 weeks, but in the presence of normal intraorbital contents. Cryptophthalmos is usually bilateral, and when it occurs with multiple other abnormalities, Fraser syndrome, which has an autosomal recessive inheritance, should be considered.
Incidence: Anophthalmia is seen in approximately 1:2,400 pregnancies.
Pathogenesis/Etiology: Primary anophthalmia/microphthalmia occurs when one or both eyes never form. It is usually associated with chromosomal abnormalities such as trisomy 13, genetic abnormalities such as CHARGE (Coloboma, Heart defects, Choanal atresia, Growth retardation, Genital, and Ear abnormalities) syndrome (CHD7), incontinentia pigmenti (IKBKG), Norrie disease (NDP), SOX2-related eye disorders, Walker–Warburg syndrome (POMT1),11–16 and over 180 syndromes.
In secondary anophthalmia/microphthalmia, the development of the eyes is initially normal but is disrupted by an insult. Etiologies include infection such as TORCH (toxoplasmosis, other, rubella, cytomegalovirus, and herpes), syphilis, Eppstein-Barr virus (EBV), parvovirus, a vascular event (e.g., Goldenhar syndrome [oculo-auriculo-vertebral spectrum]), or a toxic or metabolic event, for example, low or high vitamin A, ethanol, and retinoic acid. Table 13.1 summarizes the ocular disorder and its primary and secondary causes.
Imaging: On ultrasound (US) and MRI, microphthalmia or anophthalmia may be present when the orbital diameter is below the 5th percentile.8 Walker–Warburg syndrome, a type of congenital muscular dystrophy, comprises a Z-shaped brainstem on midline sagittal views, an occipital cephalocele, and ocular asymmetry (Fig. 13.1-3). Matthew Wood syndrome, i.e., Spear syndrome, comprises Pulmonary agenesis, Microphthalmia, and Diaphragmatic defect (PMD) or Pulmonary agenesis, Diaphragmatic defect, Anopthalmia and Cardiac anomalies (PDAC). (Fig. 13.1-4).17 Aicardi syndrome is a rare genetic malformation syndrome that can be diagnosed antenatally by the association of partial or complete absence of the corpus callosum, ocular abnormalities, and posterior fossa cyst (Fig. 13.1-5).
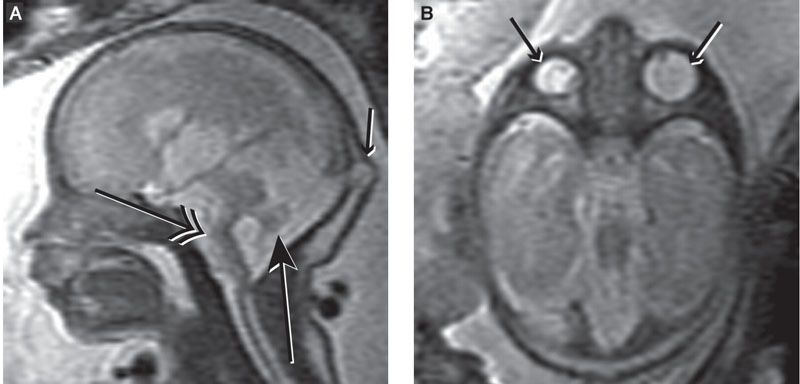
FIGURE 13.1-3: Walker–Warburg syndrome A: Sagittal T2 MRI demonstrates Z-shaped brainstem (double-headed arrow), small vermis (large arrow), and occipital cephalocele (small arrow). B: Axial MRI shows asymmetric globes (arrows). (Reproduced from Robinson AJ, Blaser S, Toi A, et al. The fetal cerebellar vermis: assessment for abnormal development by ultrasonography and magnetic resonance imaging. Ultrasound Q. 2007;23(3):211–223.)
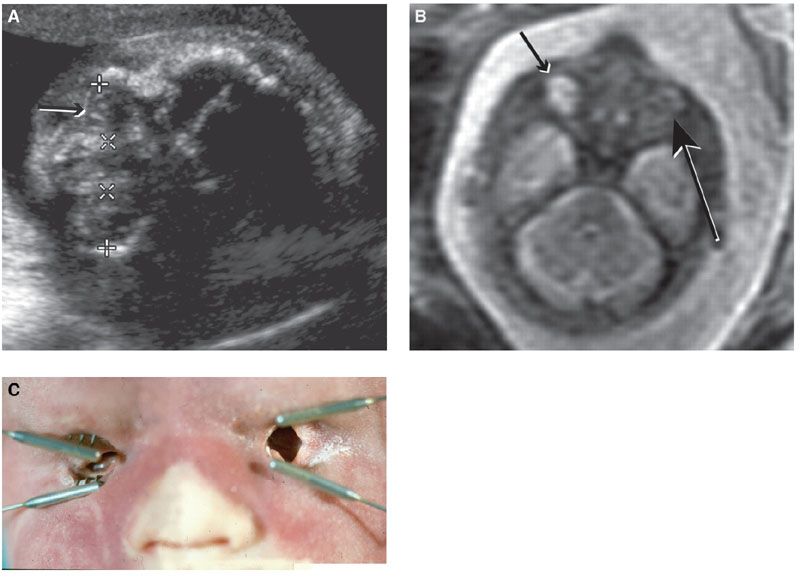
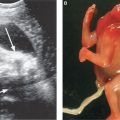
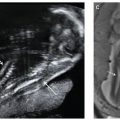
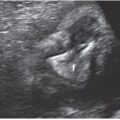
FIGURE 13.1-4: Matthew Wood syndrome. A: US biometry was delayed and there was an abnormal appearance to lens and globe, which appear echogenic (arrow). B: MRI demonstrates apparent anophthalmia (large arrow) and microphthalmia (small arrow). C: Postmortem showed absent globes. (Reproduced from Robinson AJ, Blaser S, Toi A, et al. Magnetic resonance imaging of the fetal eyes: morphologic and biometric assessment for abnormal development with ultrasonographic and clinicopathologic correlation. Pediatr Radiol. 2008;38:971–981, with permission of Springer-Verlag.)
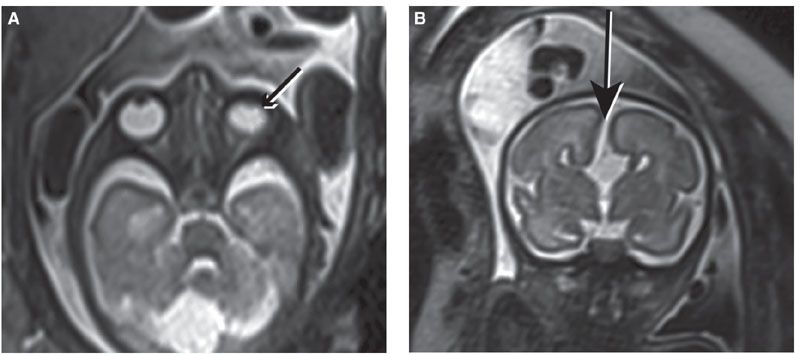
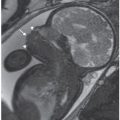
FIGURE 13.1-5: Aicardi syndrome. A: Unilateral microphthalmia (arrow). B: Callosal dysgenesis with “high-riding” third ventricle continuous with interhemispheric fissure (arrow).
HYPOTELORISM
Pathogenesis/Etiology: The craniofacial skeleton is derived from both mesoderm and neural crest cells of the mesencephalon. Its development is intimately related to forebrain development and has a similar induction mechanism. For this reason, facial skeletal abnormalities are often associated with underlying cerebral malformations (the face predicts the brain18). Typically, the craniofacial abnormalities are actually secondary to a more caudal expression of an abnormal genetic gradient that exists within the neural crest, and therefore it is actually the brain abnormality that affects the development of the craniofacial skeleton, that is, the brain predicts the face (H. Sarnat, personal communication, 2012).
During normal embryologic development, the opening for the eye forms during development of the face, when the paired nasal swellings on either side migrate medially and inferiorly and fuse with the midline frontal swelling to form the nose. If there is deficient forebrain development, with deficient development of the midline frontal swelling, the paired nasal swellings migrate more medially, and this results in primary hypotelorism. The corresponding underlying brain anomaly is often in the spectrum of holoprosencephaly,8,19 with approximately 55% of cases associated with chromosomal abnormalities, most commonly trisomy 13.5,8
In secondary hypotelorism, the defect is most frequently the result of abnormalities of the bony skull, for example, microcephaly and plagiocephaly.8
Imaging: Hypotelorism is defined as an interocular distance below the 5th percentile on US and MRI.8 Close inspection of the brain for imaging findings of holoprosencephaly is indicated (Fig. 13.1-6).
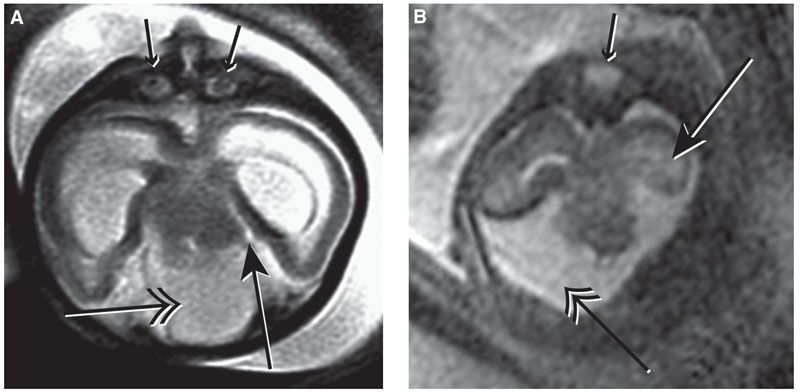
FIGURE 13.1-6: Holoprosencephaly. A: Axial MR image shows severe hypotelorism and microphthalmia (small arrows). There is a dorsal interhemispheric cyst (double-headed arrow), and the hippocampus touches the brainstem at the ambient cistern (large arrow), in keeping with lobar holoprosencephaly. B: Axial MR image shows a single small midline globe (small arrow). The hippocampus is not touching the brainstem (large arrow), and there is wide communication of the ambient cistern with the dorsal interhemispheric cyst (double-headed arrow), in keeping with alobar holoprosencephaly. (Reproduced from Robinson AJ, Blaser S, Toi A, et al. Magnetic resonance imaging of the fetal eyes: morphologic and biometric assessment for abnormal development with ultrasonographic and clinicopathologic correlation. Pediatr Radiol. 2008;38:971–981, with permission of Springer-Verlag.)
HYPERTELORISM
Pathogenesis/Etiology: In primary hypertelorism, the defect is due to deficient migration of the neural crest cells in the lamina terminalis of the prosencephalon. When this occurs, there is deficient formation of a structure known as the medial canthal ligament, and the eyes stay in their early embryonic position on the side of the face similar to lesser mammals, fish, and birds (except owls). The deficient migration of neural crest cells also typically results in associated callosal dysgenesis. Hypertelorism can result from many chromosomal anomalies and genetic syndromes,11 including median facial cleft syndrome, otherwise known as frontonasal dysplasia, which comprises hypertelorism, facial clefting, and callosal agenesis.
In secondary hypertelorism, the defect typically results from abnormalities of the skull; the most common being anterior cephalocele and craniosynostoses.11,19,20 Although typically used to describe the appearance of the skull in type II thanatophoric dysplasia, the term “cloverleaf skull” has also been used to describe the dysmorphic appearance of the skull in craniosynostosis,21,22 and several case reports have described the utility of fetal MRI in antenatal diagnosis.23–25
Imaging: Hypertelorism is defined as an interocular distance above the 95th percentile on US and MRI.8 Interocular distance may be the most reliable for making the diagnosis of hypertelorism, as by US this measurement is usually more than two standard deviations from the mean, whereas the binocular distance remains at the upper limit of normal.8 In primary hypertelorism, other anomalies are commonly identified (Fig. 13.1-7). In the presence of craniosynostosis or cephaloceles, hypertelorism may be secondary (Fig. 13.1-8).
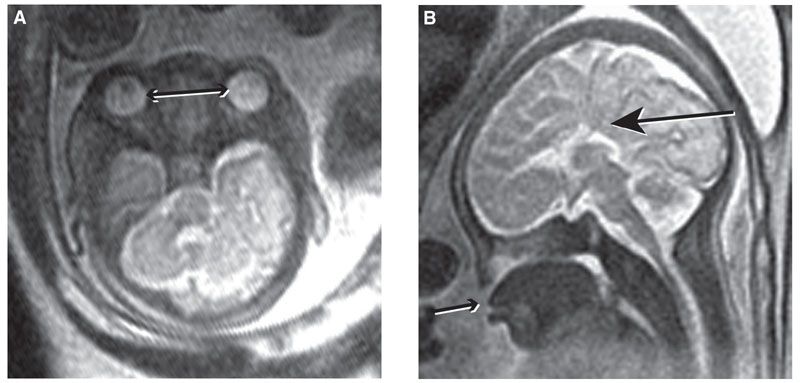
FIGURE 13.1-7: Frontonasal Dysplasia A: Axial MR image shows hypertelorism (double-headed arrow). The BOD and IOD were both greater than the 95th centile for gestational age. B: Absence of the corpus callosum (large arrow) and facial defect with absence of the hard palate separating the nasal and oral cavities with the tongue protruding through the defect (small arrow). (A, reproduced from Robinson AJ, Blaser S, Toi A, et al. Magnetic resonance imaging of the fetal eyes: morphologic and biometric assessment for abnormal development with ultrasonographic and clinicopathologic correlation. Pediatr Radiol. 2008;38:971–981, with permission of Springer-Verlag.)
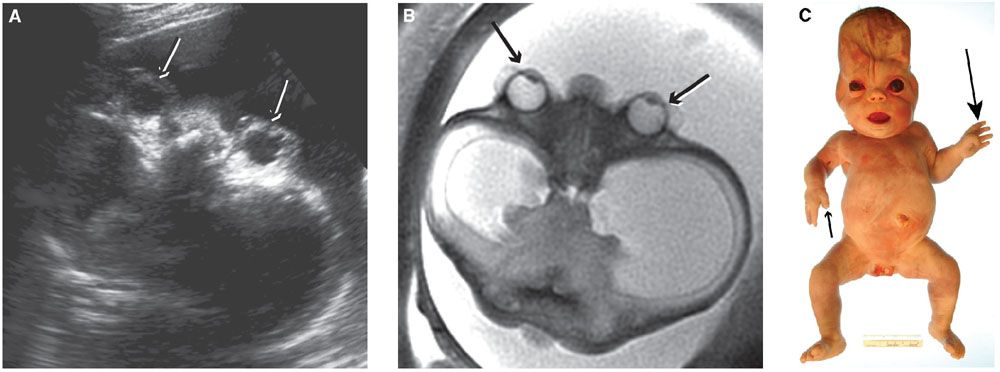
FIGURE 13.1-8: Craniosynostosis in Pfeiffer Syndrome. A:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree