Fig. 14.1
The identification of SLNs. ICG can be injected around a tumor by endoscopy prior to surgery or by the direct injection of ICG during the surgery
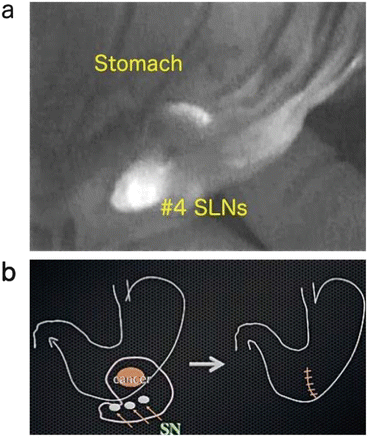
Fig. 14.2
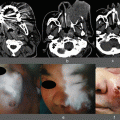
(a) Intraoperative detection of SLNs. (b) When no micrometastasis is detected in SLNs, is the partial resection of stomach acceptable?
The correct marking of the tumor lesion is essential for the laparoscopic or other minimally invasive surgery for GI tract cancers. Tattooing by India ink and a clipping procedure have been widely employed, but they present risks of dirty staining or the leakage of India ink into the abdominal cavity [5] and the dropout of clips. We developed a novel endoscopic device [6], and here we evaluated the ICG fluorescence marking method as a new way to tattoo a GI tumor.
Several studies [7–9] have reported a significant correlation between the number of lymph node (LN) metastases and the number of retrieved LNs, and the number of metastatic LNs is the main prognostic factor in patients with gastric cancers. It is thus mandatory to harvest LNs from resected specimens very precisely for the accurate determination of the clinical stage in gastric cancer.
In this study, we tested the efficacy of the IFI method for the retrieval of LNs from resected GI cancer specimens.
14.2 Materials and Methods
14.2.1 The Detection of SLNs
Fluorescence images were obtained after an injection of ICG using a charge-coupled device fluorescent endoscope with a cut filter as the detector and light-emitting xenon at 840 nm as the light source. There are two ICG injection approaches. In one approach, ICG is injected into the subserosa around the tumor. In the present study, 0.3 mL of ICG solution was injected at three points around the tumor prior to the patients’ surgeries. In the second approach, the ICG is injected intraoperatively.
The patient series in our preliminary study consisted of 22 patients with gastric cancer and 26 patients with colorectal cancer, all of whom had undergone a standard surgical resection [10]. In the open laparotomy cases, we observed the SLNs using a fluorescence imaging device (photodynamic emission [PDE] camera) after the laparotomy. In the laparoscopy-assisted gastrectomy cases, a fluorescent endoscope was used.
14.2.2 Tattooing the Tumor Region Using ICG Fluorescence
ICG injections were given to the patients undergoing preoperative gastrectomy or colectomy for early-stage stomach and colon cancers or adenomas. After the laparotomy, the stomach or colon was first observed with the naked eye, and then the PDE camera or fluorescent endoscope was used.
14.2.3 ICG Fluorescence-Assisted Ex Vivo Lymph Node Harvest in Gastric Cancer
Three timings for the ICG injection have been explored: preoperative, intraoperative, and postoperative injection. Here we focused on the ex vivo injection of ICG in 20 patients with proven gastric cancer. After resection, 0.3 mL of ICG solution was injected into four or five points of the submucosal areas. LNs were then harvested by the conventional procedure. We reexamined whether there were any LNs left behind, using the PDE camera. Lastly, we evaluated IF-labeled LNs in paraffin-embedded specimens from the patients.
14.3 Results
14.3.1 The Detection of SLNs
We observed that during the surgeries, soon after the laparotomy, the tumors were easily recognized and lymphatic channels draining from the tumor to the SLNs were clearly visible by fluorescence and the SLNs’ bighting fluorescence (Fig. 14.2a). Even the SLNs that were not fluorescent green in color could be easily and clearly visualized by the IFI imaging. The SLN detection rate in our preliminary study was 90.9 % and the mean number of SLNs was 3.6 in the gastric cancer patients, and the corresponding values in the colorectal cancer patients were 88.5 % and 2.6.
14.3.2 The Tattooing of the Tumor Region by ICG Fluorescence
After the laparotomy, we observed the tattooing areas by using the PDE camera or fluorescent endoscope (Figs. 14.3 and 14.4). In our preliminary study we combined the injection of ICG and India ink and the clipping method in an attempt to identify the marking spots without fail. However, when we injected India ink prior to the ICG injection, we could not detect the tattooing points due to the ink’s obstruction of the ICG pathway to the LNs.
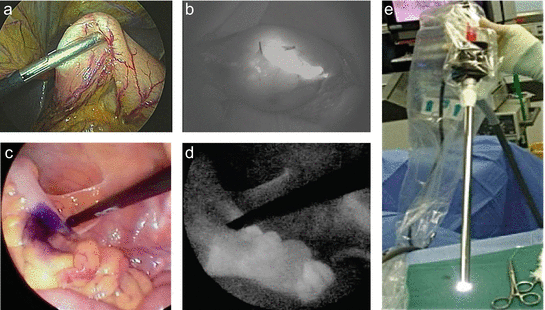
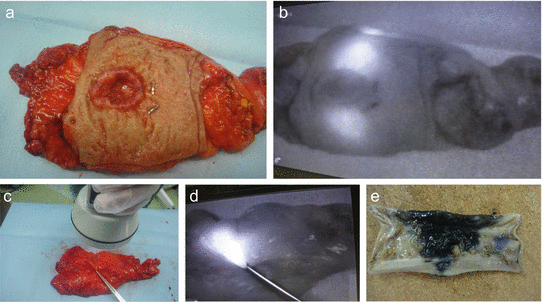

Fig. 14.3
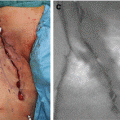
Tattooing the tumor region by ICG fluorescence in the early gastric cancer. The stomach was mobilized laparoscopically and exteriorized through a minilaparotomy incision (a). The fluorescence-marked tumor localization was clearly visualized (b). (c–e) Fluorescent laparoscopic findings during a colon surgery. The fluorescent area around the tumor (a) was clearly visualized by fluorescent endoscopy (e). After marking using pyoktanin dye (d), a segmental colectomy was performed

Fig. 14.4
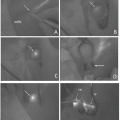
Rectum cancer with tattooing by ICG. (a, b) The marking spot cannot be seen by the naked eye, but fluorescent spots were clearly observed at the upper and lower sides of the tumor by the PDE camera (see Fig. 14.4b). (c) The observation of fluorescent-tattoo spot by PDE camera. (d) The marking spot cannot be seen by the naked eye (Fig. 14.4c), but fluorescent spots were clearly detected (serous side, Fig. 14.4d). (e) Resected specimen tattooed by India ink (the case shown in Fig. 14.4e)
14.3.3 ICG Fluorescence-Assisted Ex Vivo Lymph Node Harvest in Gastric Cancer
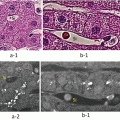
Soon after the gastrectomies, we injected ICG at several points in submucosal areas around the tumors in resected specimen. A few minutes later, the lymphatic ducts and lymph nodes that generated fluorescence were detectable by the PDE camera (Fig. 14.5d–f). LNs were found by IFI even after the conventional harvesting of LNs.
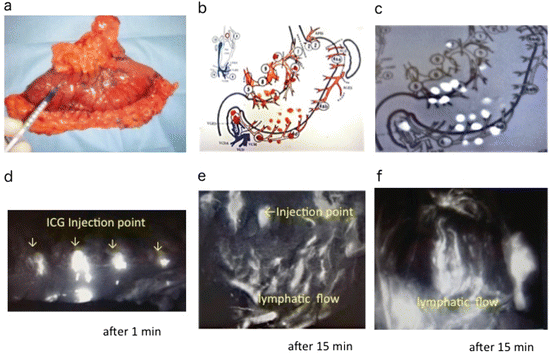

Fig. 14.5




ICG fluorescence-assisted ex vivo lymph node harvest in GI tract cancers. (a–c) Ex vivo ICG injection (gastric cancer). ICG was injected directly into the subserosa. A conventional LN harvest (b) and additional LN harvest (c) were then performed. (d–f) Ex vivo ICG injection (colon cancer). After the injection of ICG, the lymphatic flows appeared gradually. One min (d) and 15 min (e, f) after the injection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree