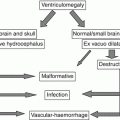
Preplacental hypoxia
Hypoxic environment
Maternal cardiovascular diseases
Maternal anemia, infection, or chronic inflammation
Utero-placental hypoxia
Abnormal placenta implantation
Preeclampsia
Postplacental hypoxia
Diminished uterine artery flow
Progressive fetal cardiac failure
Vascular malformations (i.e.VGAM)
Twin-to-twin transfusion syndrome
Genetic anomalies
9.1.1 Focal Ischemic Lesions on Arterial Basis
Focal ischemic lesions on arterial basis may present as vascular territory distribution: middle cerebral arteries in the majority of cases (Fig. 9.1), but other main cerebral artery distribution can be affected as well. In some fetal cases, however, a clear vascular territory distribution cannot be determined (Fig. 9.2). Other times vascular watershed territories result involved the most (Figs. 9.3, 9.4, and 9.5). It is important to underline how initial focal ischemic lesions may often evolve into hemorrhagic transformation, leading to a hemorrhagic necrosis appearance (Fig. 9.6). For this reason, it is not always easy to distinguish between pure ischemic arterial lesion and focal hemorrhagic lesion, especially in subacute and chronic phase.
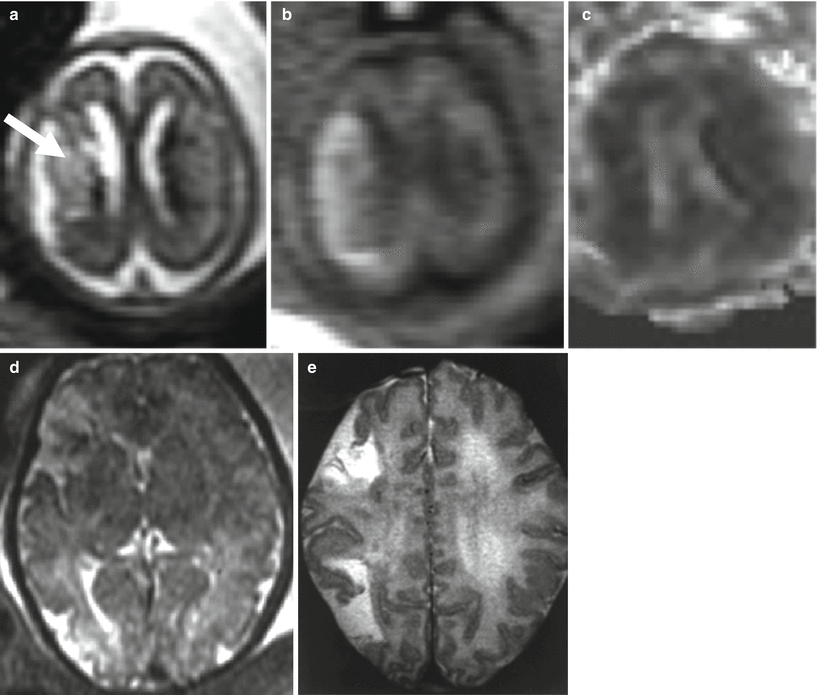
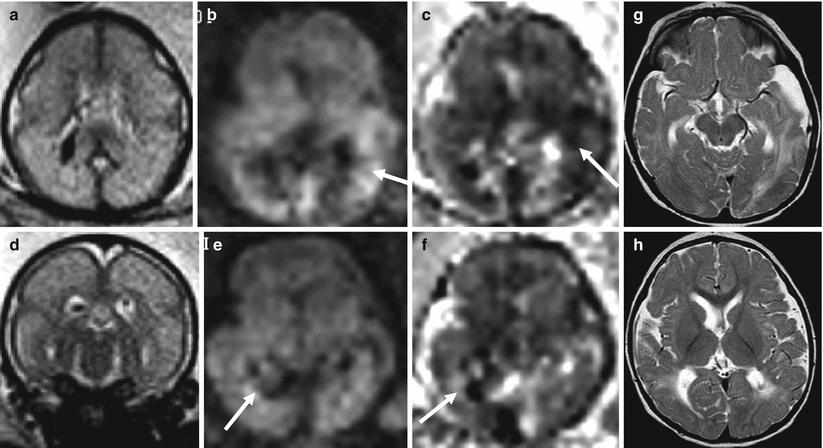
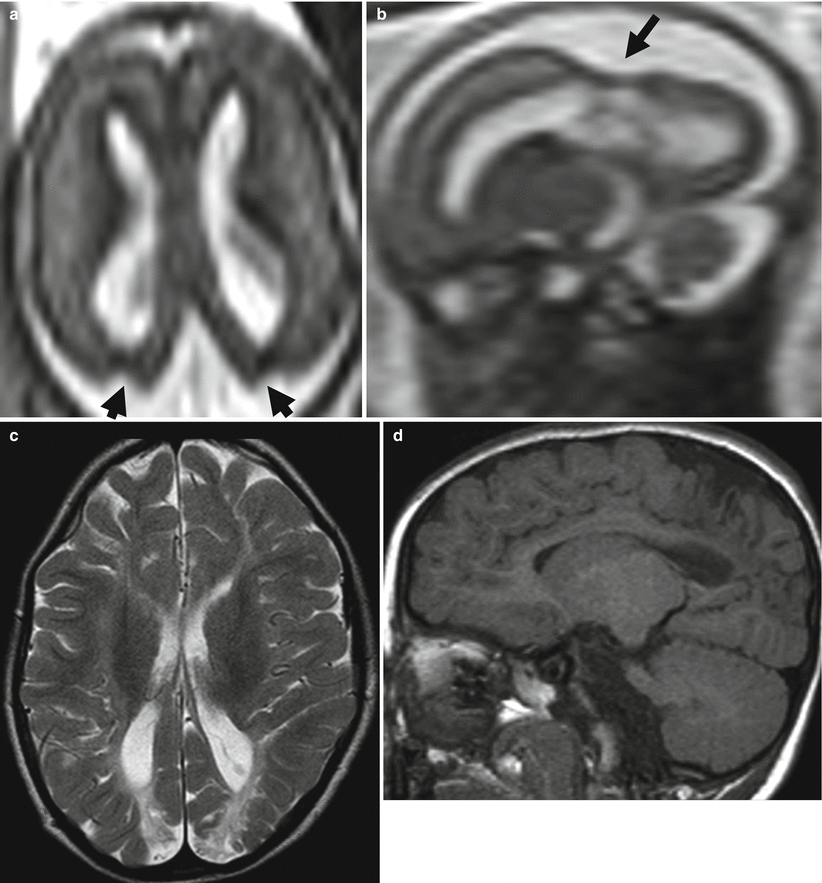
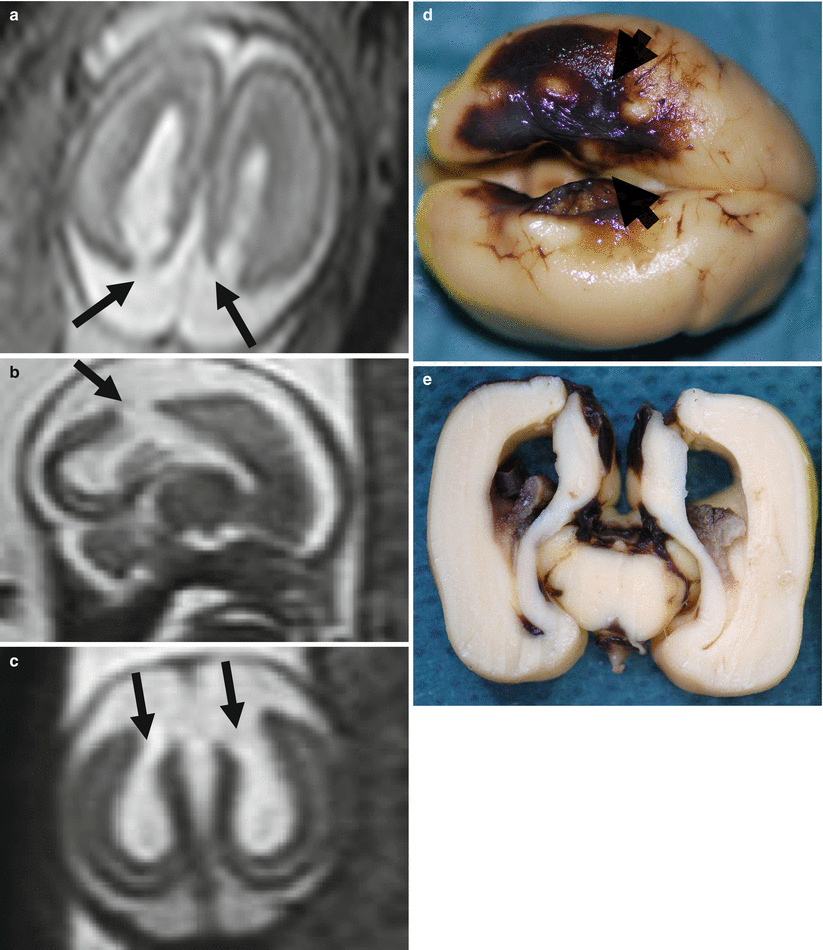
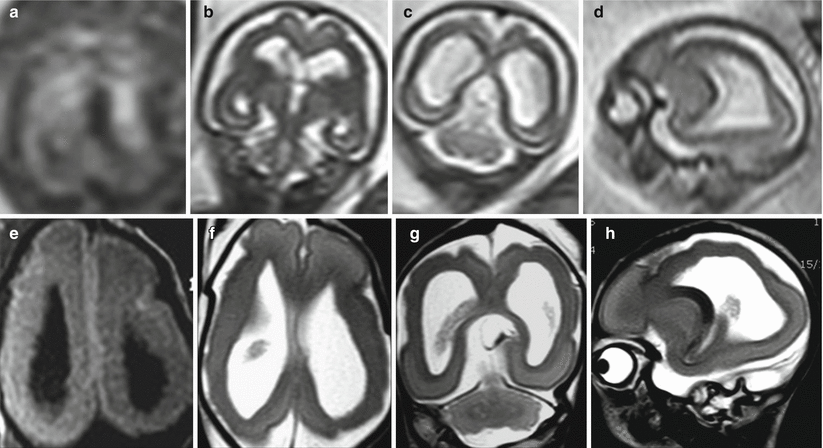
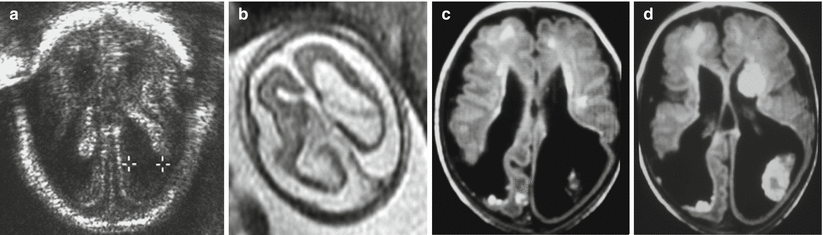

Fig. 9.1
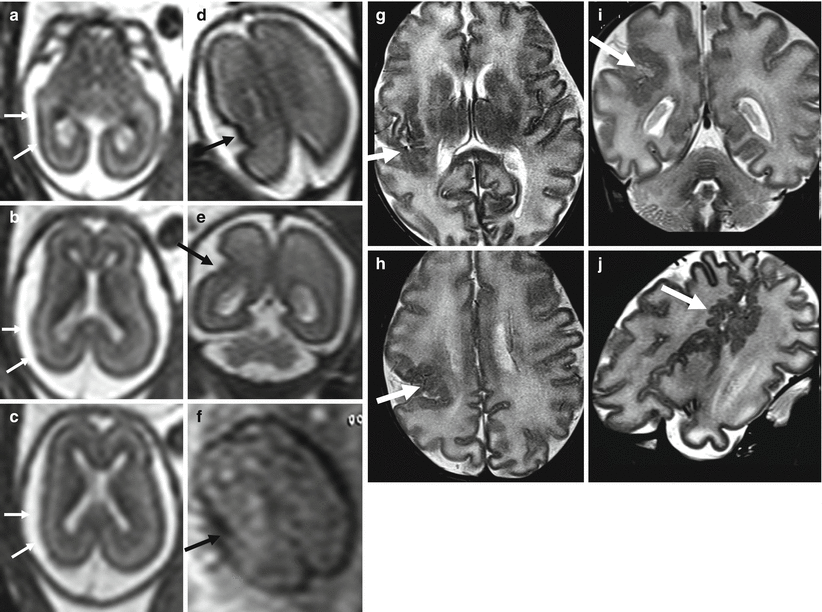
Middle cerebral artery infarct. Fetal MR at 21 GW shows a large frontal–parietal unilateral area with abnormally increased T2-weighted signal (arrow a) and diffusion restriction (b, c) compatible with acute–subacute ischemic damage in the putative territory of middle cerebral artery. Fetal MR study of a different case performed at 34 GW after reduction of spontaneous fetal movement in utero; it shows multiple foci of ischemic lesion in anterior and posterior territory of MCA (d); these findings were confirmed after birth (e)

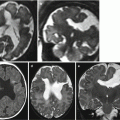
Fig. 9.2
Multiple ischemic lesions in a survivor monochorionic twin with TTTS. Fetal MR study at 25 GW shows diffuse brain edema (a, d) with multiple areas of diffusion restriction (b, c, e, f arrows). Postnatal MR study at 1 year of age (g, h) confirms multiple focal and mostly subcortical lesions

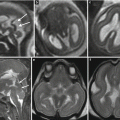
Fig. 9.3
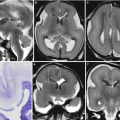
Watershed vascular territory infarction. Fetal MR study at GW 21 (a, b) discloses a marked reduction of parenchymal thickness in the parasagittal parieto-occipital areas of both hemispheres with disruption of subplate and intermediate zone, compatible with the chronic stage of a watershed territory infarction (arrows). The postnatal MR study in another case at 2 years of age (c, d) demonstrates the atrophic sequelae in the cortical–subcortical parenchyma of parieto-occipital region bilaterally

Fig. 9.4
Watershed territory infarction. Fetal MR study at GW 21 (a–c) shows a transmantle parenchymal disruption involving both superficial and deep layers (arrows). These aspects were confirmed at brain autopsy (d arrowheads, e) with cortical necrosis and hemorrhagic components well appreciable

Fig. 9.5
Watershed territory infarction. Fetal MR study at GW 20 (a–d) shows a diffuse thinning of parenchymal layers in the parasagittal regions and in the superficial territories of both middle cerebral arteries. Cortical mantle exhibits a diffuse hypointense signal on T2-weighted images and an hyperintense signal on T1-weighted images (a). These aspects were confirmed on fetal MR autopsy (e–h) and resulted as cortical-subcortical necrosis at pathology

Fig. 9.6
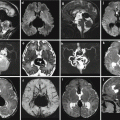
Ischemic lesions with hemorrhagic components. US study at 19 GW (a) and fetal MR study at 21 GW (b) demonstrate thinning of the parenchymal layers in the posterior aspect of both hemispheres; the distorted and hyperechoic choroid plexus (a) is compatible with the presence of hemorrhagic debris. Similar postnatal case (c, d) demonstrates as well a diffuse parenchymal disruption in the occipital-parietal regions, with hemorrhagic components within ventricles and brain parenchyma
When the onset time of lesions is within hours or few days range before imaging, the detection of ischemic areas mainly relays on the use of DWI (Fig. 9.7); although affected by technical limitations and by motion artifacts, DWI sequence, especially when repeated, is able to highlight areas of increased signal and decrease ADC (Figs. 9.1 and 9.7). Acute lesion detection is a rare event [6], and in most of cases, MR imaging is prompted by death of one twin in monochorionic twin pregnancies or by complications during LASER treatment of placental anastomoses in monochorionic twins [7] (see chapter 10).
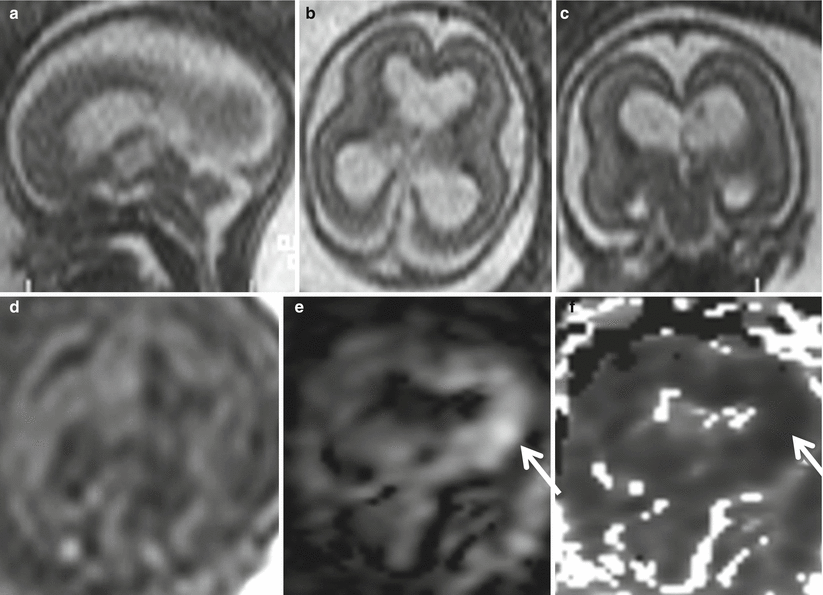

Fig. 9.7
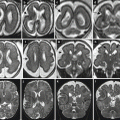
Acute–subacute ischemic lesion. Fetal MR study at GW 20 shows moderate ventriculomegaly (15 mm atrial width) and mild macrocrania on both T2 (a–c)- and T1 (d)-weighted images. On DWI (e) and ADC (f) images, a focal diffusion restriction is evident in the left periventricular region (arrows). After pregnancy termination, pathology revealed necrosis in periventricular parenchyma
In most cases, focal ischemic lesions are detected in subacute or chronic phase as ultrasound hyperechogenic (subacute phase) or hypoechogenic (chronic malacia or focal atrophy) areas. In subacute phase (some days from insult onset), DWI signal could be still [8] increased, but the most important finding is represented by blurring of normal cerebral mantle layering, moderate signal increase, and mantle swelling on ultrafast T2-weighted images . In the transition period between subacute (days to 2 weeks) and chronic phase (from weeks to months), the lesion area could undergo some heterogeneous T2-weighted signal decrease and T1-weighted signal increase, due to hemorrhagic necrosis (Figs. 9.4 and 9.5).
Focal lesions evolve into chronic end-stage sequelae: focal lobe volume reduction, focal ventricle enlargement, cyst and malacyc areas formation, and nodular or linear hemosiderin deposits are all typical features of such kind of evolution (Figs. 9.5 and 9.6). End-stage sequelae may appear faster than it usually happens in adult brain focal ischemia, even in few weeks [9]. For this reason, it is very difficult to determine the age of chronic lesions only on MRI basis (Fig. 9.9). The poor capability of reparative gliosis and the high water content of fetal brain may give reason of the fast tissular liquefaction leading to malacic–cystic areas.
When focal lesions occur before 24 GW, there is the possibility that, in addition to the abovementioned sequelae, also malformations may arise (i.e., polymicrogyria, schizencephaly, heterotopias) (Fig. 9.8). In this regard, ischemia affecting neuronal pool in active proliferation, migration, and organization phase may be followed by focal development of cortical malformations, that could be considered a sort of reparative phenomenon proper of the early fetal age [10, 11]. Finally, false-negative fetal MR studies may obviously occur, especially in the evaluation of the brain of a survived or supposed normal twin in a TTTS setting (Fig. 9.9). It has to be underlined however how a focal ischemic lesion may occur at any stage of gestation and a negative fetal MR study at midgestation cannot totally predict the post-natal outcome.