A recent study performed on 162 fetuses from 21 to 40 weeks of gestational age confirmed that the customarily used sectional planes for sonography and MR imaging measurements of the lateral ventricles are in close agreement [17].
On the basis of these findings, MR does not have a role in assessing the size of the ventricular trigones over and above sonography, but with two exceptions: (1) when the fetal head position and calvarial ossification may hamper the visualization of the entire ventricular system on sonography and (2) when there is a limitation in visualizing the lateral ventricle closest to the sonography probe due to the near-field effect, which may be of relevance in cases of asymmetric ventriculomegaly.
7.2 Etiology-Based Classification
Ventriculomegaly is often considered the “tip of the iceberg,” because it is a finding frequently associated with numerous cerebral anomalies.
The causes of ventriculomegaly are multifold, but they may be divided into three main categories: obstructive, dysgenesis, or destructive causes (Table 7.1).
Table 7.1
Main causes of ventriculomegaly
Obstructive | Dysgenesis | Destructive |
|---|---|---|
Chiari II malformation | Agenesis of the corpus callosum | Intracranial hemorrhage |
Dandy–Walker malformation | Septo-optic dysplasia | Periventricular leukomalacia |
Aqueductal stenosis | Holoprosencephaly | Hydranencephaly |
Mass lesions | Schizencephaly | Infection |
Obstructive causes lead to enlargement of a normally formed supratentorial ventricular system.
The ventricles, cortex, and midline structures are normally developed but altered in appearance due to excess fluid within the ventricular system. Examination of the posterior fossa often reveals the abnormality causing obstruction. In the setting of a normal posterior fossa, aqueductal stenosis is the presumed diagnosis (Figs. 7.2 and 7.3). It should be noted, however, that the hydrocephalus associated with aqueductal stenosis is often massive and may make identification of most structures challenging.
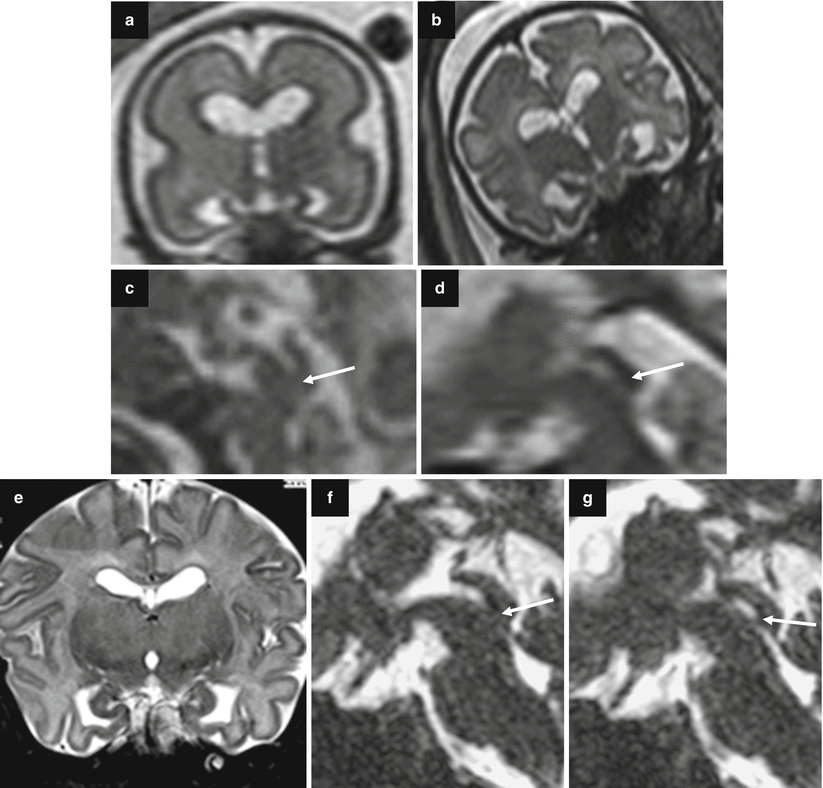
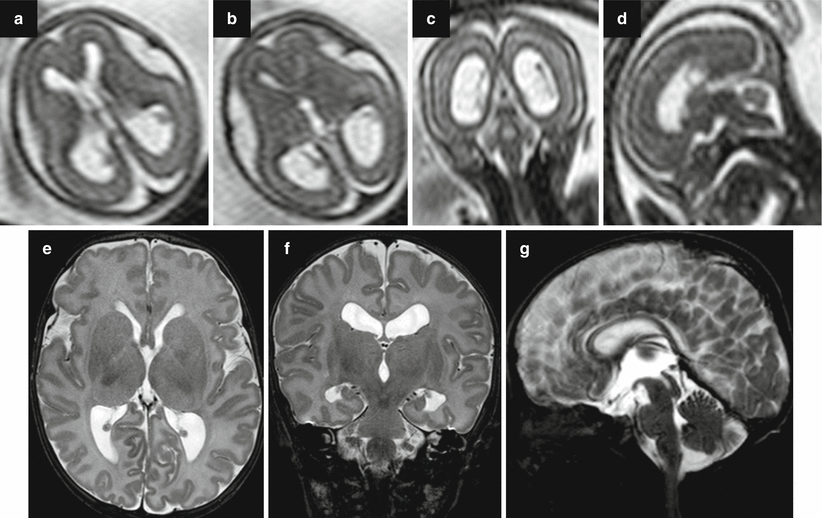

Fig. 7.2
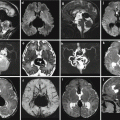
Aqueductal stenosis. Fetal US study at 24 GW showed bilateral isolated ventriculomegaly (atrial size 13 and 14 mm). Fetal MR performed at 24 GW (a, c) and at 32 GW (b, d) confirmed the slight ventriculomegaly and showed an aqueductal stenosis (arrows c, d). Neonatal MRI study at 40 GW confirmed the ventriculomegaly (e) and showed the aqueductal stenosis (arrows f, g)

Fig. 7.3
Aqueductal stenosis. Fetal MRI performed at 21 + 5 GW showed a mild ventriculomegaly (a–c) (atrial width 11–11 mm) and suspected aqueductal stenosis (d). The postnatal MRI at 40 GW confirmed the dilatation of lateral ventricles (e, f) and aqueductal stenosis (g)
Fetal infections, mainly toxoplasmosis and cytomegalovirus, may cause ventriculomegaly usually as a consequence of ventriculitis with debris or synechiae obstructing the aqueduct and subsequent obliteration. Infections are more frequently found in cases of severe ventriculomegaly which develop in late pregnancy. The rate of infection is 10 e 20 % in severe ventriculomegaly [18, 19] and 1 e 5 % in mild ventriculomegaly [8, 18–20]. A search for possible fetal infections should be always performed when ventriculomegaly is recognized.
Fetal ventriculomegaly can be caused by mass lesions (Figs. 7.4 and 7.5).
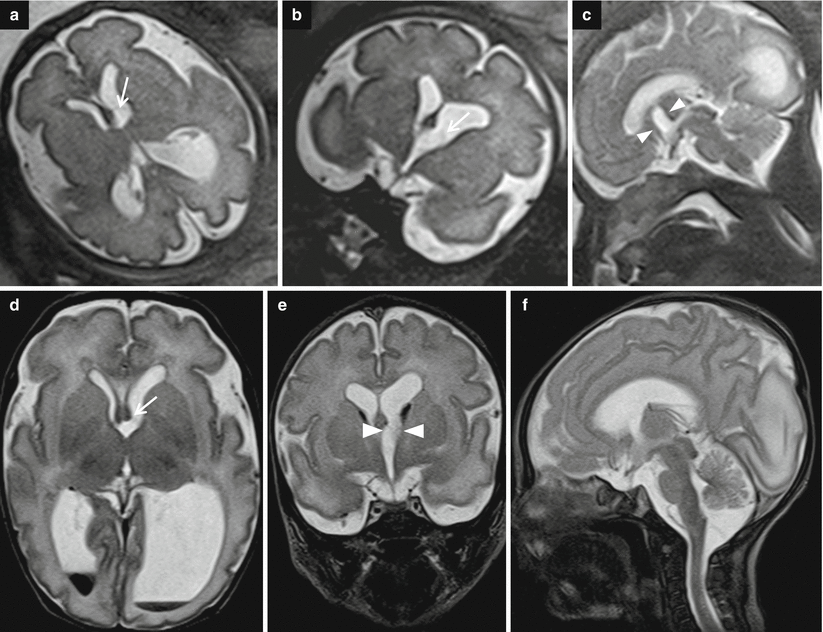
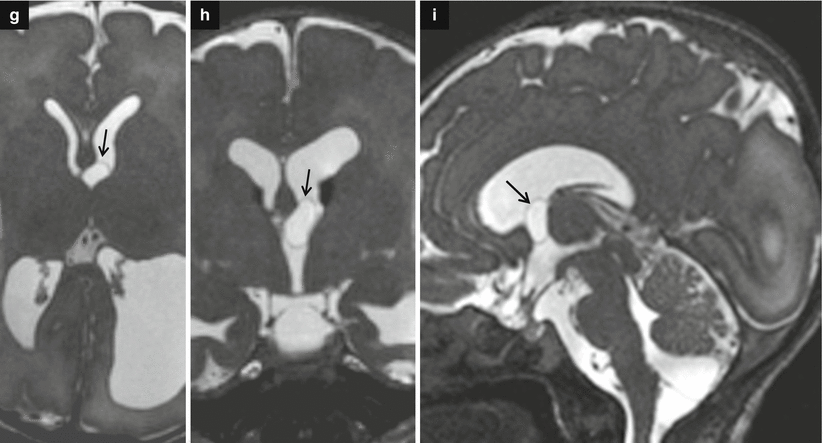



Fig. 7.4
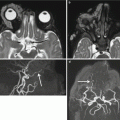
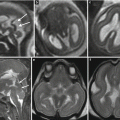
Asymmetric ventriculomegaly, severe on the left (left trigone = 18 mm). Fetal brain MRI at 31,4 GW (a–c). The foramina of Monro are clearly asymmetric: in the larger one, a faint linear hypointensity is barely visible (arrows a, b) suggesting the presence of a cystic lesion. The columns of the fornix result as slightly compressed and mildly displaced upward and to the right side supporting the hypothesis of a cystic lesion in the left foramen of Monro (arrowheads c). Postnatal brain MRI chronological age: 4 days old; post-conceptual age: 33,5 GW, T2 TSE images (d–f). The foramina of Monro (d, e) are still asymmetric: again, there is a questionable linear hypointensity in the left (larger) foramen of Monro (white arrow and white arrowheads), but no cystic lesion is clearly discernible. Blood/CSF level in both occipital horns due to germinal matrix haemorrhage (GMH) and subsequent intra-ventricular haemorrhage (IVH) are evident. On CISS images (g–i) a cystic lesion in the left foramen of Monro (black arrows) is clearly detectable, explaining the asymmetric prenatal ventriculomegaly and the asymmetric postnatal hydrocephalus. Note the faint hyperintensity of the left occipital lobe white matter (f, i), due to interstitial edema. There are some septa in the suprasellar cistern and in the cisterna magna, probably secondary to the IVH (Courtesy L. Pinelli, Dept. of Neuroradiology, Spedali Civili, Brescia)

Fig. 7.5
Acqueductal stenosis due to a small dermoid lesion of lamina quadrigemina not identifiable in fetal MRI; fetal MRI false negative. Fetal MR performed at 21, 6 GW (a–d) shows ventriculomegaly; the sylvian aqueduct region is apparently unremarkable and no CNS abnormalities are seen. Neonatal MR depicted a small dermoid lesion of lamina quadrigemina, hypointense on T2-weighted images (e, g), and hyperintense in T1-weighted images (f) that causes an aqueductal stenosis
Mild and severe ventriculomegaly can be due to the presence of a clot within the aqueduct, as a consequence of germinal matrix hemorrhage (Fig. 7.6); in this condition the ventriculomegaly can be due also to destructive damage of cerebral hemispheres as ex vacuo effect.

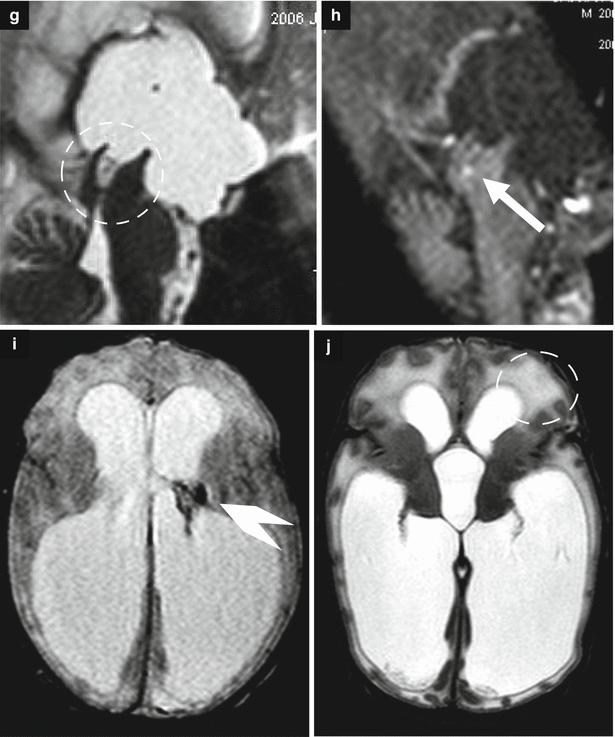


Fig. 7.6
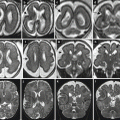
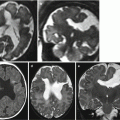
Obstructive ventriculomegaly. Fetal MRI performed at 36 GW (a–f) in a male fetus showed severe ventriculomegaly with intraventricular hemorrhage and a clot within the aqueduct (arrows b, c) and the lateral ventricle (arrowhead d) and suspected aqueductal stenosis (dotted circle a). The periventricular white matter is hyperintense on T2-weighted images (dotted circle e) due to transependymal reabsorption, in the same ROI the ADC is increased (dotted circle f). The neonatal MRI performed at birth (g–j) confirmed severe ventriculomegaly with intraventricular hemorrhage (arrowhead i) and demonstrates a clot within the aqueduct (arrow h) that results clearly stenotic (dotted circle g). The periventricular white matter is hyperintense for transependymal reabsorption (dotted circle j)
Destructive changes result from an in utero insult to a normally formed ventricular system and brain; therefore, midline structures are always present, if not totally disrupted as well. With destructive causes, ventricular enlargement is often a result of ex vacuo dilatation, although specific imaging findings vary depending on the acuity and magnitude of the insult (Figs. 7.6, 7.7, 7.8, 7.9, and 7.10). Clues to this diagnosis are symmetrically or asymmetrically enlarged portions of the ventricles, nodularity of the ventricular wall or septa indicating prior hemorrhage, altered signal and morphology, or variable amounts of absent cortical plate.
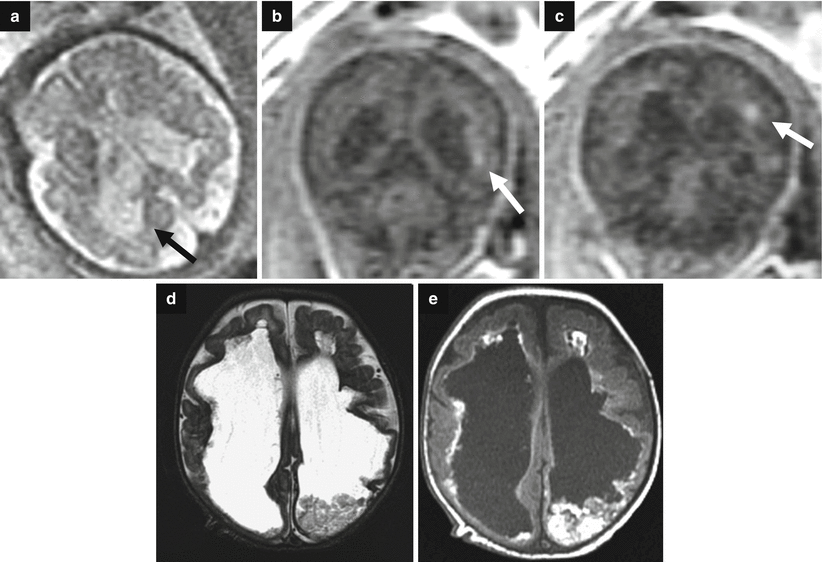
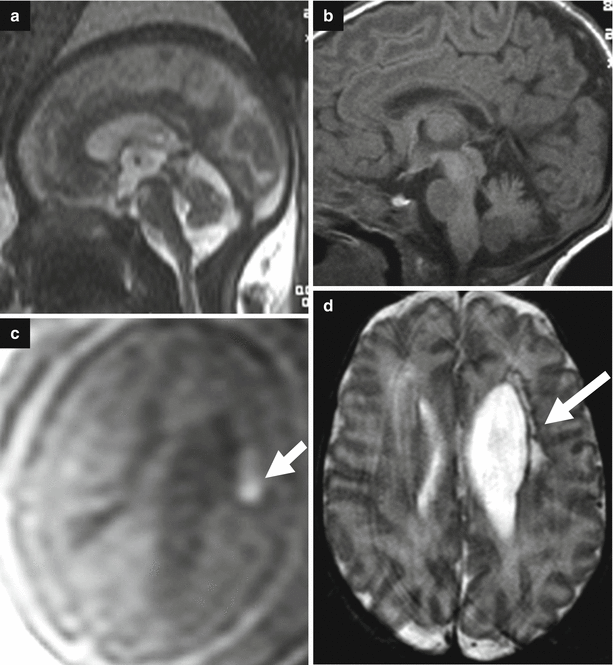
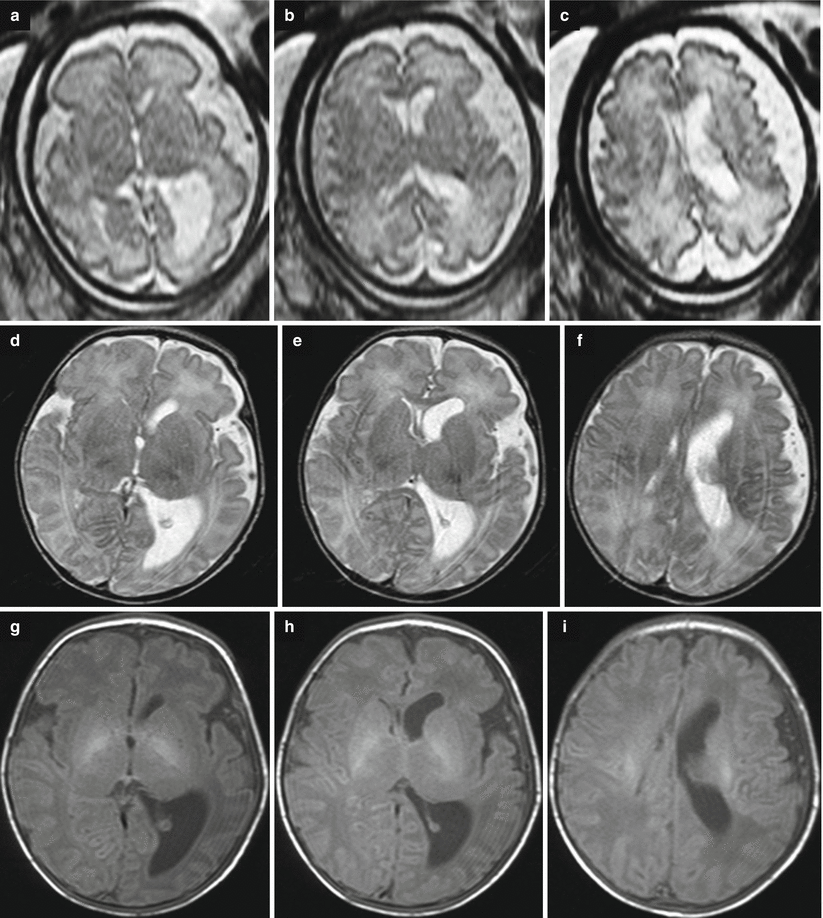
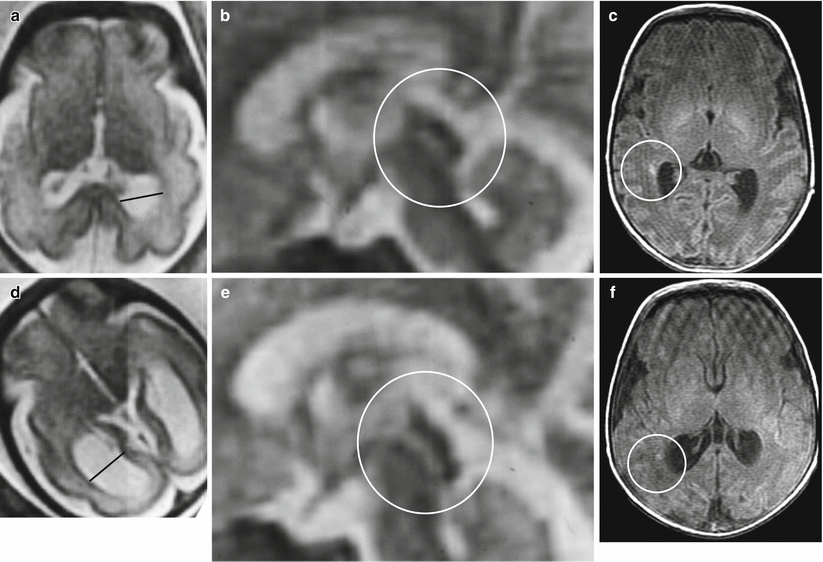

Fig. 7.7
Destructive ventriculomegaly. Fetal MRI performed at 34 GW (a–c) and neonatal MRI (d, e) show bilateral severe ventriculomegaly due to diffuse brain damage. On fetal MRI focal periventricular T1-weighted image hyperintensities are depicted (white arrows b, c). Periventricular margins are irregular with diffuse parenchymal thinning (arrow a). The neonatal MRI shows an evolution in severe and diffuse hemorrhagic periventricular leukomalacia. A clot is still present in the left lateral ventricular cavity

Fig. 7.8
Destructive ventriculomegaly. Fetal MR performed at 33 GW (a, c) in a female fetus and neonatal MRI (b, d) shows ventriculomegaly (11 and 17 mm atrial width) and hemorrhagic periventricular infarction with an intraventricular hemorrhage. In fetal MRI the T1-weighted image depicted the hyperintensity of blood (white arrow c). The neonatal MRI confirms the periventricular chronic malacic lesion with the correspondent ventricular enlargement (white arrow d)

Fig. 7.9
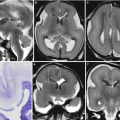
Destructive ventriculomegaly. Fetal MR performed at 32 GW shows unilateral ventriculomegaly with a thin rim hypointense in T2-weighted images, probably of hemorrhagic nature (a–c). Neonatal MRI confirms the unilateral ventriculomegaly and left periencephalic subarachnoid spaces enlargement probably caused by an hemorrhagic event (d–i)

Fig. 7.10
Destructive ventriculomegaly. Fetal MR studies of 30 GW in monochorionic twins (a, b, d, e) One twin (a, b) had a borderline mild unilateral ventriculomegaly (black line: atrial width 13 mm); the aqueduct seemed normal (circle). At birth (c) the neonatal MRI depicted periventricular punctate lesions hyperintense on T1-weighted images associated with mild periventricular leukomalacia (circle). The other co-twin (d–e) had a bilateral asymmetric ventriculomegaly with one atrial width of 17 mm (black line); the aqueduct was normal (circle). At the neonatal MRI (f) mild unilateral periventricular leukomalacia with periventricular punctate lesions was observed (circle)
Dysgenesis causes frequently show enlargement of the ventricular system as a result of cerebral maldevelopment. Clues to this diagnosis are abnormalities in ventricular shape or orientation and a spectrum of absent midline structures ranging from the septum pellucidum alone (Fig. 7.16) to complete absence of all midline divisional structures creating a fused appearance of many structures or as opposite an exaggerated separation. Examples of ventriculomegaly consequent to brain malformation are reported in Figs. 7.15, 7.16, and 7.17.
The incidence of chromosomal abnormalities is high (>15 %) in both mild and severe ventriculomegaly in the presence of an associated structural anomaly [21]. In cases of isolated ventriculomegaly, on the contrary, the association with abnormal karyotype is relatively low [18, 22]. The incidence of abnormal karyotype in fetuses with isolated mild ventriculomegaly is a controversial issue: the reported percentages range from 0 to 26 % with an average value of 2.7 % [7, 8, 18, 19, 23, 24]. The wide variation in results may well depend on maternal age which is not reported in most studies. Trisomy 21 and trisomy 18 are the chromosomal abnormalities more often associated to fetal ventriculomegaly.
In the setting of mild ventriculomegaly without associated CNS structural anomaly, abnormal karyotype, or other identifiable cause, the clinical outcome is favorable. As the degree of ventriculomegaly increases, there is increased association with other abnormalities [25].
7.3 Ventriculomegaly and Associated Structural Abnormalities
Ventriculomegaly may be associated with neural and extraneural anomalies. There is a clear relationship between the degree of ventriculomegaly, the rate of progression of ventriculomegaly, and the risk of other brain abnormalities, as reported by Griffiths PD and colleagues in a study on 147 fetuses with fetal ventriculomegaly diagnosed on sonography (Table 7.2) [14].
Table 7.2
Risk of detecting brain abnormalities on fetal MR relative to degree of ventriculomegaly of the fetus
Mild VM (%) | Moderate VM (%) | Severe VM (%) | |
|---|---|---|---|
Overall risk of another brain abnormality | 6 | 14 | 57 |
Risk of another brain abnormality in 20–24 GW group | 6 | 17 | 22 |
Risk of another brain abnormality in 25 GW + group | 6 | 9
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|