(1)
Department of Radiology and Neuroradiology, Children’s Hospital V. Buzzi, Milan, Italy
(2)
Department of Neuroradiology, Fondazione IRCCS Cà Granda, Ospedale Maggiore Policlinico, Milan, Italy
The eye forms directly from the embryonic neural tube starting from the fourth week of gestation, under the regulation of several genes and proteins [1]. Malformation of the eye therefore may be isolated, accompany other complex malformations of the nervous system, or may be a part of multisystem developmental abnormalities, due to genetic, environmental, or both genetic and environmental factors. Numerous ocular and orbital anomalies can be recognized and diagnosed on clinical evaluation, but radiologic assessment is often crucial for the differential diagnosis, for the definition of the prognosis and for treatment planning. Moreover, the evaluation of the eye and orbit is a reasonable expectation as part of a detailed fetal US or MRI scan, particularly in the setting of suspected CNS malformations, so that some developmental abnormalities can be recognized also from midgestation onward [2].
Knowledge of the embryology of orbital structures and familiarity with their anatomy and their normal development during fetal and neonatal period are essential for understanding many congenital diseases that affect this anatomical compartment.
Because ocular abnormalities may be bilateral and symmetric and thus easy to overlook particularly during fetal life, measurement of orbital/ocular biometry and evaluation of ocular morphology and position are mandatory when reviewing any imaging examination.
5.1 Embryology
The optic vesicle arises from the forebrain vesicle on approximately day 22 of gestation and enlarges rapidly growing toward the surface ectoderm and transforming into a goblet-shaped cup by means of invagination of its distal central part, called retinal disk, and of its inferior surface, called choroidal fissure. Between day 31 and day 33, surface ectoderm begins thickening and forms lens placode, which invaginates up to detach and transform into the lens vesicle during the fifth week [1, 3]. At the end of the fifth week, surrounding mesenchyma differentiates to form the inner choroid and outer sclera (comparable to the pia mater and dura mater of the brain); the mesenchymal tissue that enters the optic cup via the choroidal fissure gives increase to the hyaloid vessels and to the vitreous body; the hyaloid artery, running on the ventral surface of the optic stalk and cup, provides nourishment for growth of the retina and lens; as the lens matures, the portion of hyaloid artery which traverses the vitreous degenerates and a hyaloid canal (Cloquet’s canal) is left behind [1]. The neural retina differentiates between the sixth week and the eighth month of fetal life, and the axons from retinal neurons extend to the brain through the optic stalk, which transforms into the optic nerve [1]. Oculomotor nerves appear during the fifth week, and the primordia of the extraocular muscles, originating from mesenchyma surrounding the optic vesicle, appear by the sixth week. By the seventh week, eyelid primordia develop on the edges of the future cornea, and the mesenchyme between the surface ectoderm and the lens differentiate into the structures of the anterior chamber [4].
5.2 Biometric/Morphometric Assessment
During fetal life, ocular biometry using US is well established and measurement of binocular (BOD), interocular (IOD), and ocular (OD) distances is made according to the bony landmarks of the medial and lateral orbital walls in the axial plain [5]. Also, the fetal lens may be visualized by intravaginal US as early as 14 weeks’ gestation as an hypoechoic structure with thin echogenic margins in the anterior aspect of the globe, but sometimes, the impossibility to obtain the correct angle of insonation may prevent its evaluation [2].
Normal growth charts of the eyes and orbit using MRI are now available too [2]; measurement of BOD, IOD, and OD is made on axial or coronal planes, considering as landmarks the medial and lateral margins of the vitreous, which is hyperintense on T2-weighted images (Fig. 5.1). MRI is also useful in evaluating ocular morphology, which physiologically changes during fetal life, resembling a “drop” in the axial images obtained at earlier gestational ages and gradually assuming a spherical shape with fetal growth (Fig. 5.2). For this reason, some authors have provided data also on the anteroposterior diameter of the globes [6]. The hyaloid artery is identifiable until the 24th week, as thin hypointense line that connects the posterior portion of the lens with the dorsal margin of the globe, at which time begins to disappear. Even the lens becomes progressively better defined, and from the 27th week, it is possible to appreciate the anterior chamber as fine hyperintense rhyme. The small size of optic nerves in fetal life and the low spatial resolution of fetal MRI allow only to assess if they are present or not, but it’s not possible to judge their signal or size.
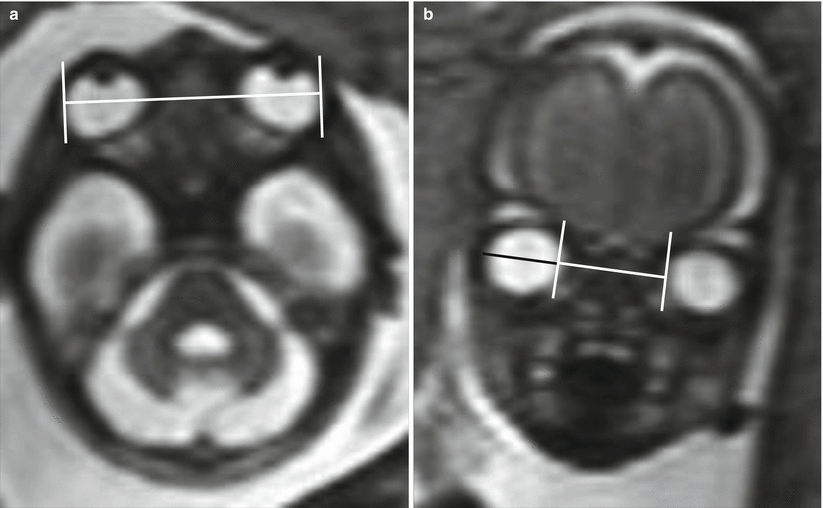


Fig. 5.1
Axial and coronal T2-weighted images show the measurement of ocular biometric parameters. (a) Binocular diameter. (b) Interocular diameter (in white) and ocular diameter (in black)

Fig. 5.2
Axial (top) and sagittal (bottom) T2-weighted images show the changes in the morphology of the eyeball with advancing gestational age
On neonatal imaging, the globe morphology and position should be evaluated too, as a detailed analysis of extraocular muscles, orbital fat and vasculature, and periorbital soft tissues to identify any infiltration or mass is mandatory. Optic nerves, third, fourth, and sixth cranial nerves should be identified and assessed for atrophy or compression. To assess for proptosis on CT or MRI axial imaging, we could draw a transverse line between the two zygomatic processes and then another perpendicular line from this to the anterior margin of the globe, which should measure less than 21 mm. Proptosis may also be defined as an eyeball whose anteroposterior diameter is placed, for less than one-third, posteriorly to the line joining the medial and lateral canthus of the orbit [2] (Fig. 5.3).
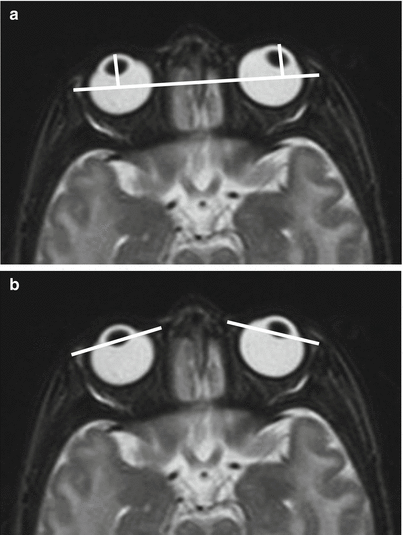

Fig. 5.3
Assessment for proptosis on axial T2-weighted MR imaging: two methods. (a). The perpendicular lines from the anterior margin of each globe to another line drawn between the two zygomatic processes should measure, in normal subjects, less than 21 mm. (b). The anteroposterior diameter of the globe should be placed, for more than one-third, posteriorly to the line joining the medial and lateral canthus of the orbit
5.3 Hypotelorism and Hypertelorism
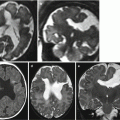
Hypotelorism refers to an IOD below the fifth percentile. Primary form is due to excessive migration of nasal swellings before fusion with midline frontal swelling and is often associated with CNS malformations in the spectrum of holoprosencephaly (Fig. 5.4). Hypotelorism is genetically determined, and in 55 % of cases, it is due to chromosomal abnormalities, among which trisomy 13 is the most commonly involved [7] (Fig. 5.5). On fetal MRI, the evaluation of the IOD is useful particularly in the cases of septum pellucidum agenesis of nature to be defined, in which the eventual detection of hypotelorism can help to exclude the lesional nature of this findings. Secondary hypotelorism is generally determined by cranial bone abnormalities such as microcephaly or craniosynostosis [8]; the latter is often responsible also for complex orbital morphological changes (Fig. 5.6).
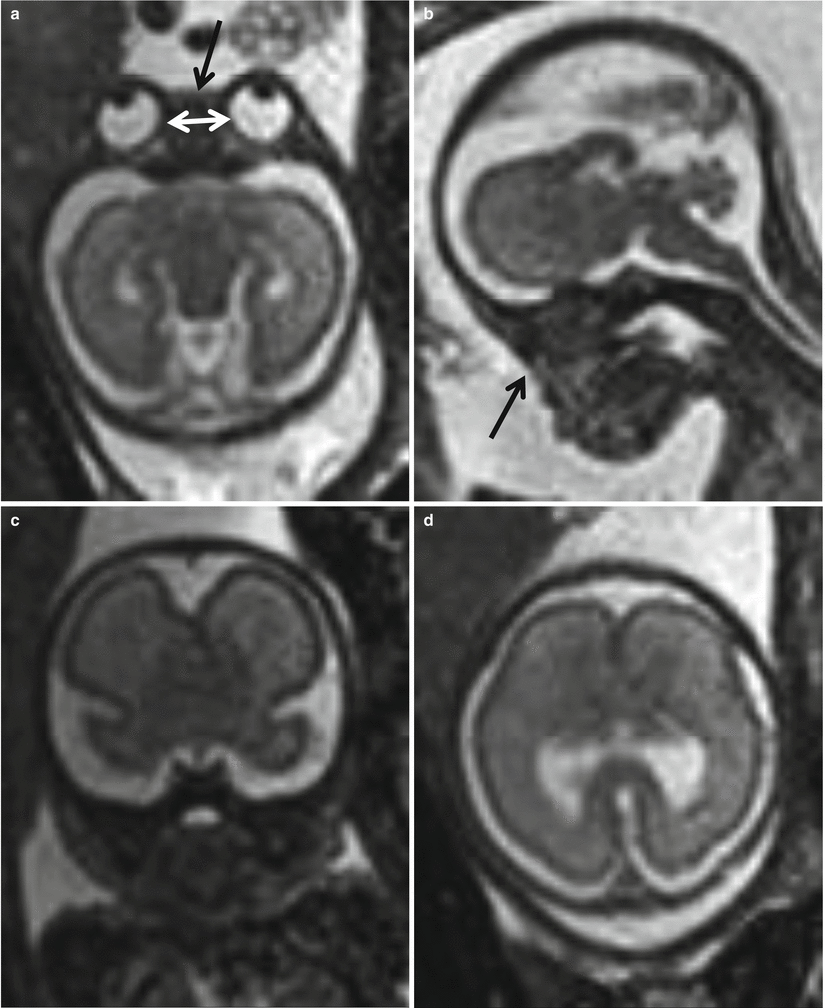
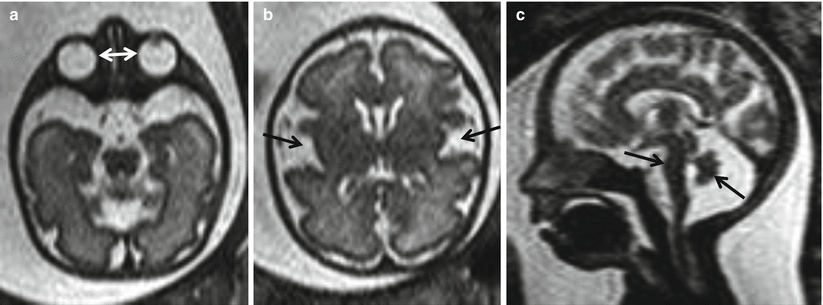
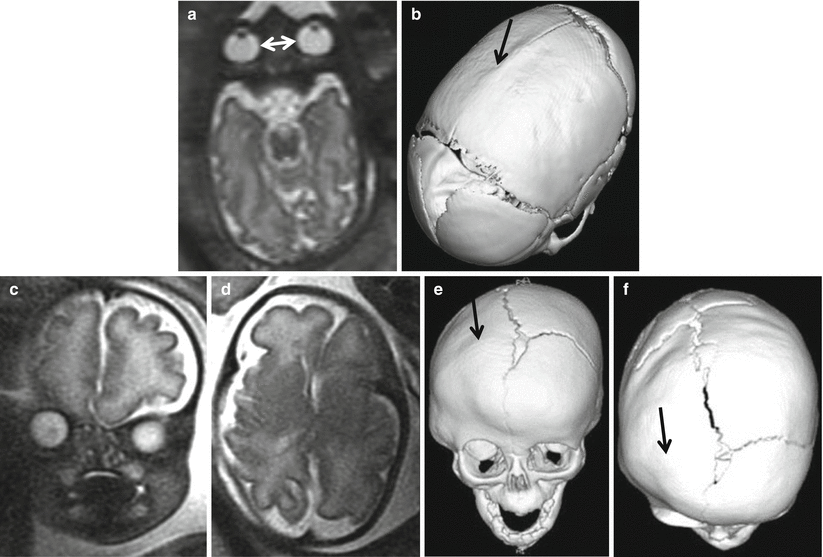

Fig. 5.4
Hypotelorism in a 25 GW fetus with holoprosencephaly and facial malformation. Axial and sagittal T2-weighted images (a, b) show hypotelorism (white arrows) and absent nasal pyramid (black arrows). Coronal and axial T2-weighted images (c, d) show fusion of frontal lobes with missing frontal horns of lateral ventricles

Fig. 5.5
32 GW syndromic fetus. Axial T2-weighted image (a) shows hypotelorism (white arrows). Axial T2-weighted image (b) shows abnormal morphology of silvian scissures (arrows). Sagittal T2-weighted image (c) shows wide cisterna magna with vermian and pontine hypoplasia (arrows), associated with mild micrognathia

Fig. 5.6
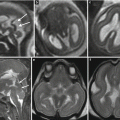
Two cases of craniosynostosis. 34 GW fetus: axial T2-weighted image (a) shows scaphocephaly and hypotelorism (white arrows); postnatal CT 3D reconstruction (b) demonstrate premature closure of the sagittal suture (arrow). 33 GW fetus: coronal and axial T2-weighted images (c, d) highlight abnormal conformation of the skull and asymmetry of the orbits. CT scan performed after birth (e, f) shows early fusion of the right coronal suture (arrows), resulting in orbital volume reduction on the same side and greater development of the contralateral frontal bone and orbit
Hypertelorism refers to an IOD above the 95th percentile. Primary form occurs if the nasal swellings do not migrate far enough medially and inferiorly and therefore often appears in conjunction with varying degrees of facial clefts and CNS abnormalities, particularly at the level of the midline, such as agenesis of the corpus callosum (Fig. 5.7). It may occur in association with various syndromes and chromosomal abnormalities. Secondary hypertelorism may be the consequence of several pathological conditions including, craniosynostosis, frontonasal encephalocele (Fig. 5.8), and dacryocystocele. In the case of craniosynostosis, especially in the context of some syndromes (e.g., in Crouzon’s syndrome), associated exophthalmos can be detected [8].
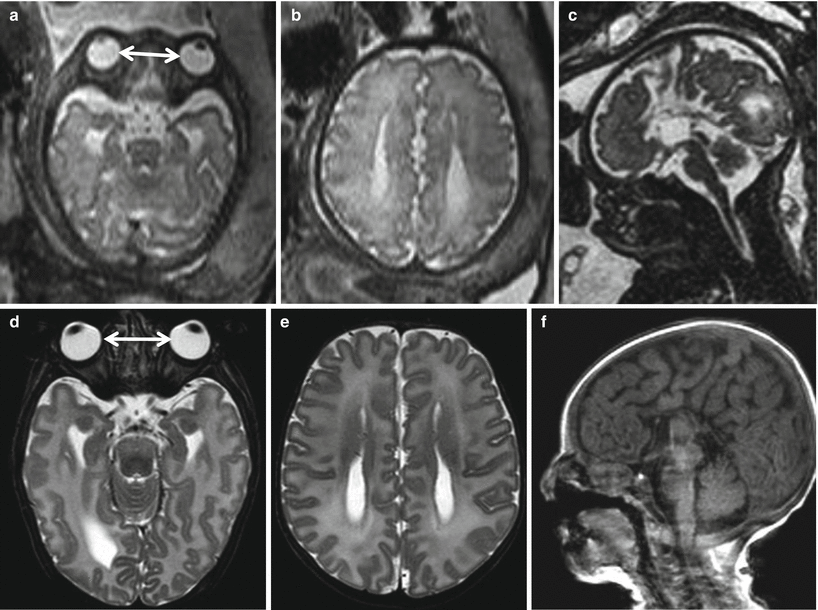
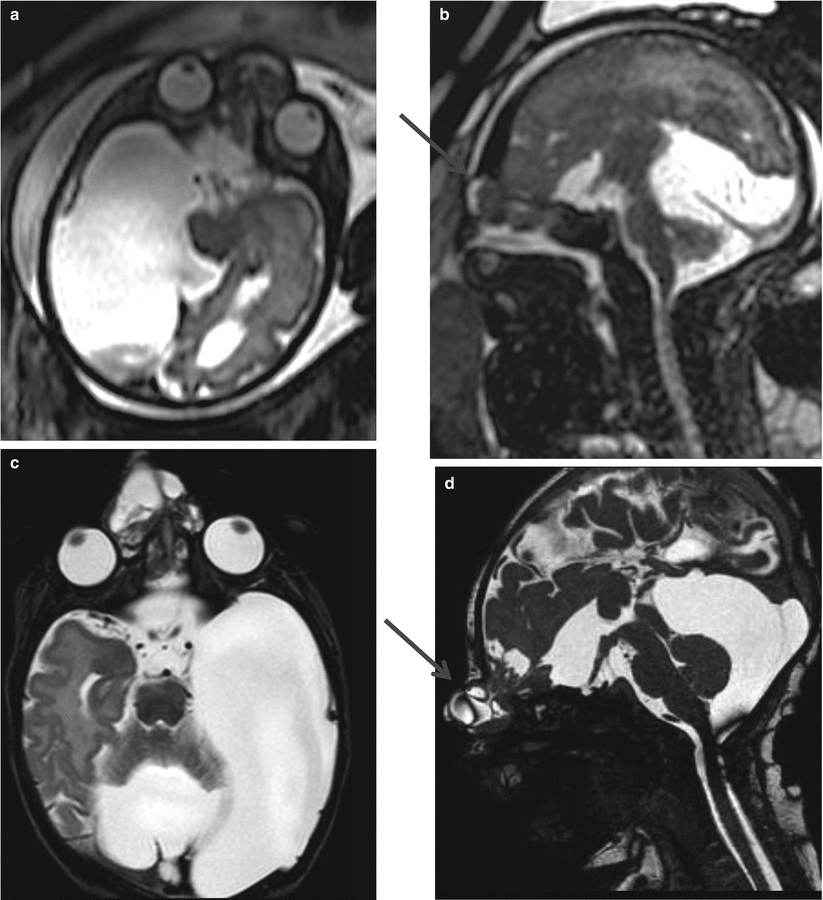

Fig. 5.7
33 GW fetus (top) and neonate (bottom) with midline CNS and midface anomalies. Axial T2-weighted images (a, d) show hypertelorism (white arrows). Axial T2-weighted images (b, e) show parallel aspect of lateral ventricles as an indirect sign of callosal agenesis. Sagittal 3D T2- (c) and T1-weighted images (f) show callosal agenesis and cleft lip and palate

Fig. 5.8
Axial T2-weighted image (a, c) and sagittal balance image (b, d) show hypertelorism and frontonasal encephalocele (arrows) in a 32 GW fetus with complex CNS malformation (top) and in the same case studied after birth (bottom)
5.4 Anophthalmia
There are three types of anophthalmia: primitive form is due to the lack of formation of the optic vesicle in the first 4 weeks of gestation; secondary form, which is lethal, results when there is failure of development of the entire anterior portion of the neural tube; and the degenerative form, due to an insult during the first 8 weeks of gestation. Anophthalmia is often observed in the context of various syndromes and chromosomal abnormalities [9]. The differential diagnosis with severe microphthalmia may be very difficult in prenatal life. Diagnosis of true anophthalmia is based on histological demonstration of the absence of neuroectodermal structures in the orbit, which is usually reduced in volume, narrow, and elongated. The globe is replaced by amorphous tissue isointense on T1 and hypointense on T2-weighted images, and the extrinsic ocular muscles may be present (Fig. 5.9).
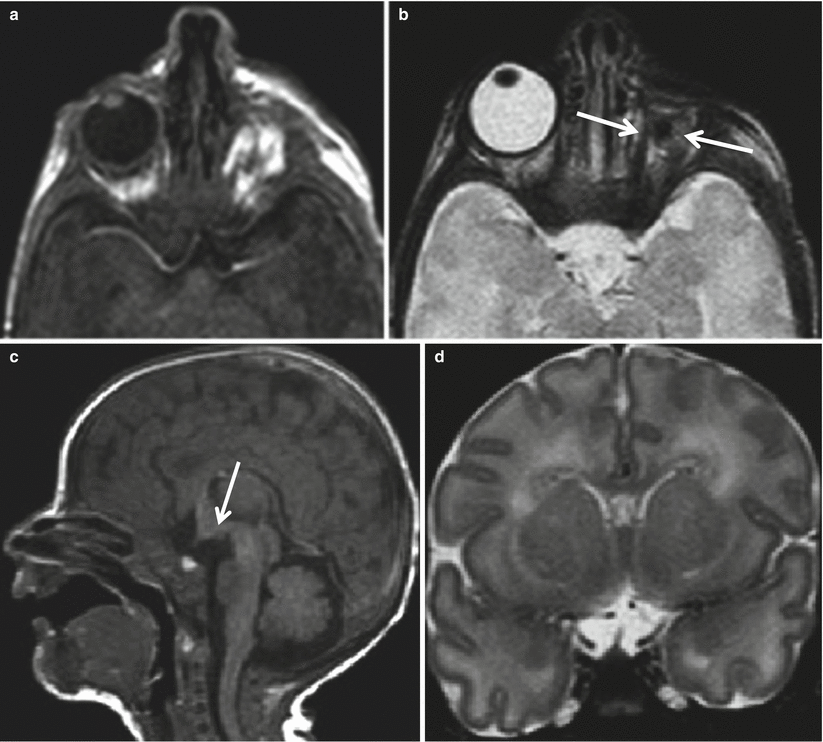

Fig. 5.9




Neonate with unilateral anophthalmia. T1-weighted and T2-weighted images (a, b) show a narrow and elongated left orbit with no ocular globe recognizable; extrinsic ocular muscles are present (arrows). Sagittal T1-weighted and coronal T2-weighted images (c, d) show hypothalamic dysmorphism (arrow) and absence of optic nerves and chiasm
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree