Fig. 1
Biodistribution of Ga-citrate in normal rats. Ga-citrate (40 MBq) was injected into rats, and images were acquired at 60 min post-injection. 67Ga-citrate SPECT images are shown in a (before purification) and b (after purification). 68Ga-citrate PET image is shown in c. Reproduced from Kumar et al. (2012)
No literature is available on early imaging times using 67Ga SPECT. In our study we compared 68Ga-citrate PET and 67Ga-citrate SPECT images at 60 min post injection in normal rats, and the results showed relatively lower background activity for the PET agent compared with the SPECT agent (Fig. 1c). This may be due to the short half-life of 68Ga (68 min), as it would have undergone nearly one half-life decay within the 60 min imaging time, compared with the SPECT agent (67Ga), which has half-life of 76 h. Therefore, the visual difference in background can be attributed to the difference in half-life between these two agents. Similarly, uptake of 68Ga by liver, cardiac blood-pool activity is much lower than for 67Ga, which may be attributed to the faster decay of 68Ga than 67Ga. The lack of uptake by the kidneys and bladder is comparable in both cases, which might explain the high vascular retention of activity in the patient study. High vascular retention may be due to high protein binding of the agent, which is to be substantiated with experimental evidence.
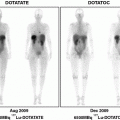
At our institution, the radiotracer is not routinely used except in pediatric patients for selected indications such as FUO. To obtain diagnostically useful images, a multipeak gamma camera equipped with a suitable medium-energy collimator is mandatory for imaging with 67Ga-citrate (Palestro 1994). 67Ga was prepared as 67Ga-citrate to facilitate solubilization, and approximately 150–200 MBq (5 mCi) was injected in patient studies; images were acquired 60 min after injection. The images indicated normal distribution of the radiotracer in liver, bone, bone marrow, and soft tissues. Bowel activity was minimal (Fig. 2a). On the other hand, biodistribution of 68Ga-citrate shows distinct high vascular activity, which is not typical of 67Ga-citrate images. 68Ga-citrate showed moderate hepatic uptake and mild bone marrow activity. Bowel activity was absent (Fig. 2b) (Nanni et al. 2010).
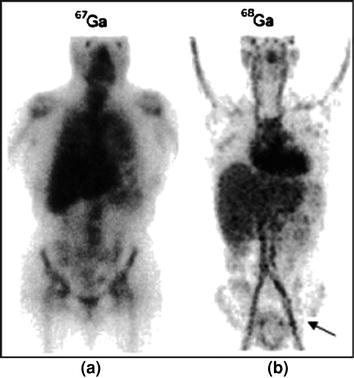

Fig. 2
Physiologic biodistribution of 67Ga-citrate whole-body scintigraphy and 68Ga-citrate PET/CT. Intense uptake of 67Ga-citrate is seen in the liver, blood pool, and kidneys (a). Significantly less uptake of 68Ga-citrate is seen in the liver, and kidneys but very high vascular activity (arrow) (b). Reproduced with permission (Nanni et al. 2010)
Background activity of 68Ga-citrate in the thorax and upper abdomen at 60 min post injection suggests possible interference with detecting lesions in these regions but may also indicate suitability for imaging the lower abdomen and the extremities. The short half-life of 68Ga (68 min) may be advantageous in terms of low dose to patients, but is disadvantageous for use of this radionuclide for longer periods of study. In our institution, we are performing clinical evaluation of this agent for imaging cellulitis and abscesses.
4.2 68Ga-Citrate PET for Imaging Staph A Infection in an Animal Model
In experimentally induced Staph A infection in an animal model, Kumar et al. (2009, 2012) demonstrated avid uptake of 68Ga-citrate at infected lesions within 10 min post injection, but focal intense uptake (SUVmax) at 30 min (Fig. 3a). They also demonstrated that the SUVmax at the target increased in a
time-dependent manner for up to 6 h post injection, with concomitant decrease in cardiac blood pool activity (Fig. 3b). Liver and bowel activity decreased up to 90 min, then stabilized. Bone marrow and growth plate uptake at joints showed moderately high uptake over the period of study. Muscular uptake decreased steadily with increased Target/Muscle (T/M) ratios over the entire period of the study from 3.7 to 8.5.
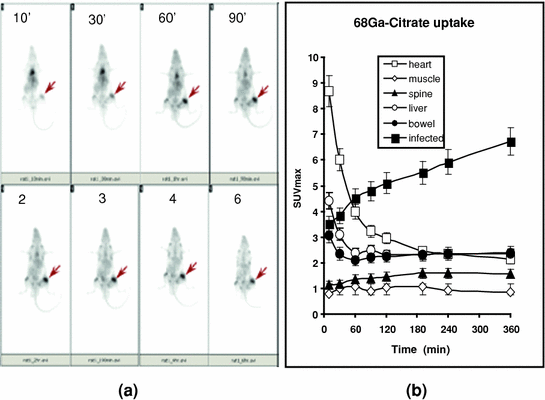

Fig. 3
68Ga-citrate uptake by Staph A infection in a rat model. 68Ga-citrate (15 MBq) was injected into an anesthetized rat, and images were acquired at different time intervals (a). Infection lesions are shown by the arrows. SUVmax was calculated for each time point over infected lesions and different organs, and the values are plotted in b. Mean ± SD was calculated (n = 5) and plotted in the graph. Reproduced from Kumar et al. (2012)
4.3 68Ga-Citrate for Diagnostic PET Imaging of Osteomyelitis and Follow-Up Treatment
Available literature indicates a number of parameters for imaging infection using different imaging modalities such as bone scanning, labeled white blood cell scanning, and MRI with sensitivity and specificity of 82–25%, 84–80% (21–60% for axial skeleton), and 84–60%, respectively (Ma et al. 1997; Termaat et al. 2005). According to results presented by Nanni et al. (2010), the performance of 68Ga-citrate PET/CT was not really superior to that of a conventional imaging diagnostic flow chart (reporting slightly higher sensitivity and comparable specificity, but with data from limited evaluation). Nevertheless, the following advantages were clearly indicated: simple and fast diagnostic procedure, no false positivity in cases of bone implants, and low dosimetry due to short half-life. The short half-life of 68Ga-citrate, although an advantage from a dosimetric point of view, could be considered a drawback at the same time, because it does not allow the long uptake time typical of 67Ga-citrate scintigraphy. However, their final results showed that a short uptake time was long enough to visualize a pathologic process, although a longer time would have guaranteed higher contrast due to reduction of background (Gelrud et al. 1974). In most patients with bone infection, they reported relatively low SUVmax, in comparison with background. Positive 68Ga PET/CT scans presented mean SUVmax of 4.4 ± 1.8. SUVmax was 3.9 ± 1.8 (range 1.7–8.0) for acute osteomyelitis, 5.5 ± 2.0 (range 4.0–7.0) for chronic osteomyelitis, and 5.8 ± 2.0 (range 3.7–8.6) for diskitis (Nanni et al. 2010). Although they used visual criteria to decide on all positive findings (tracer uptake significantly higher than background), most patients had SUVmax between 2 and 4, a value that certainly does not clearly highlight the infected area at first glance on the maximum intensity projection image. Therefore, they reviewed each scan carefully, slice by slice, and had one false-positive result from study of a total of 31 patients, which on further examination turned out to be a tumor lesion. This event was predictable because 67Ga-citrate has long been used as a marker for imaging tumors (Bombardieri et al. 2003).
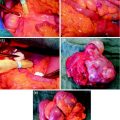
The importance of functional imaging is not limited to diagnosis of infection but extends also to surgical planning. Three-dimensional reconstruction images obtained with 68Ga-citrate PET/CT was shown to be very useful in a patient affected by acute osteomyelitis before and after surgical curettage, confirming complete response to antibiotic therapy (Fig. 4). Similarly, 68Ga-citrate PET/CT has shown avid uptake of the agent before surgery, which resolved completely after surgical curettage and local antibiotic therapy in a patient affected by acute osteomyelitis of left tibial implant (Fig. 5). Fusion of 68Ga-citrate PET images and CT images provides the surgeon with an accurate and detailed basis to better plan the operation and possibly improve patient outcome. They studied biopsy samples in all patients, but reported that only 11 of 30 biopsy samples (37%) were diagnostic. They found 4 false-positive scans, 23 true-positive scans, 13 true-negative scans, and no false-negative scans, resulting in sensitivity of 100%, specificity of 76%, positive predictive value of 85%, negative predictive value of 100%, with overall accuracy of 90%. They did not find significant tracer uptake in uninfected bone implants (Nanni et al. 2010).
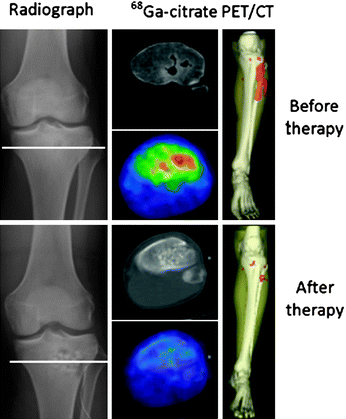
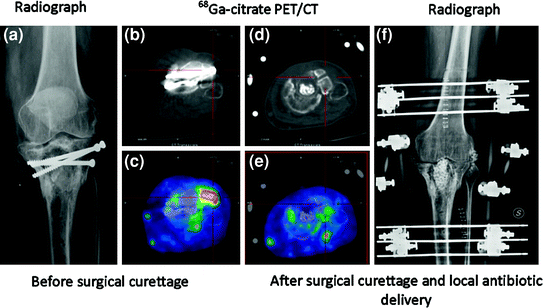

Fig. 4
Comparison of 68Ga-citrate PET/CT before and after surgical curettage in a patient affected by acute osteomyelitis. On right, three-dimensional reconstruction shows bone infection (red area) also involving surrounding soft tissue. After therapy, no uptake is evident, confirming complete response. Reproduced from Nanni et al. (2010)

Fig. 5
Comparison of 68Ga-citrate PET/CT before and after surgical curettage in patient affected by acute osteomyelitis of left tibial implant. Radiograph before therapy (a), CT scan before therapy (b), 68Ga-citrate PET/CT scan before therapy (c), CT scan after therapy (d), 68Ga -citrate PET/CT scan after therapy (e), and radiograph after therapy (f). c Focus of increased 68Ga-citrate uptake in lateral side of left tibial plate close to metal implant is consistent with acute osteomyelitis (SUVmax 3.1). e After surgical curettage and local antibiotic delivery, tracer uptake completely normalizes. After 1 year of follow-up, patient was still free from pain. Reproduced from Nanni et al. (2010)
It is widely believed that 18F-FDG is the more standard in vivo marker of bone infection. 18F-FDG is sensitive but has the great limitation of giving positive results in patients with bone prosthesis, even if there is no infection or mobilization (Gravius et al. 2010). In the population study by Nanni et al. (2010), many patients had different types of prostheses or bone implants, but 68Ga-citrate was positive only in cases of infection. However, direct comparison of the performance of the two tracers is important, and no more comments can be made without such a comparison.
In summary, their preliminary work with 68Ga-citrate PET/CT evaluated the sensitivity, specificity, positive predictive value, negative predictive value, and overall accuracy of 68Ga-citrate PET/CT in a population of patients with suspected bone infection. Their study did not include recently operated patients or patients with recent bone fractures. Patients with bone implants showed significant artifacts on CT images but no false-positive results in the corresponding PET series, which they attributed to a correction of the View-Point iterative algorithm. Interestingly, no false-negative results were found and the negative predictive value of 68Ga-citrate PET/CT was therefore quite high. This finding was confirmed by a previous preclinical publication (Makinen et al. 2005), but they indicated that the main limitation of this study is the lack of a gold standard to validate the PET/CT results—a long follow-up—which is the only reliable approach due to the cited limitations of other diagnostic procedures. They concluded “68Ga-Citrate PET/CT is a new diagnostic tool that can be considered in the flow chart of patients with bone infection. However, more experience is required to further validate these results” (Nanni et al. 2010).
4.4 68Ga-Citrate PET/CT for Imaging of Discitis, Cellulitis, and Abscess
Diagnosing discitis has been a challenge for a long time. However, vertebral lesions are diagnosed more distinctly as an intense focal uptake of 18F-FDG than any other agent (Stumpe et al. 2000), although this is expensive to perform. MRI has shown considerable success in diagnosing discitis, as shown by the area of abnormal signal in L5–S1, equivocally consistent with infective discitis, in Fig. 6. However, it is again very expensive to perform on a routine basis. 68Ga-citrate PET/CT, which is very cost-effective and uses an easily available agent, has been shown to define discitis as focal area of increased tracer uptake consistent with inflammation (Fig. 6b). In the past, 99mTc-labeled ciprofloxacin (Infecton) has been successful, as it defined the lesions associated with discitis as hot area with soft tissue extension in L2 and L3 in Fig. 7a. Conventional imaging agents such as 99mTc-HMPAO-labeled leukocyte study had limited value in imaging discitis, as it defines a large photopenic area of defect which is hard to diagnose (Fig. 7b).
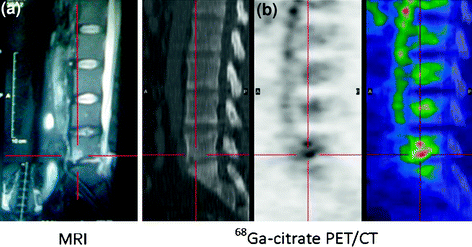
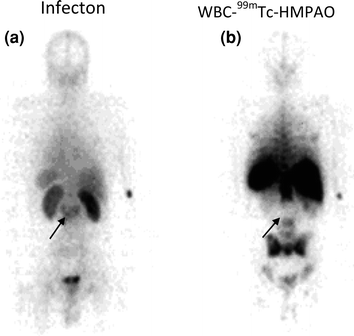

Fig. 6
Comparison of MRI and 68Ga-citrate PET/CT in patient with discitis. a MRI shows area of abnormal signal in L5–S1 that is not unequivocally consistent with infective discitis (MRI). b 68Ga-citrate PET/CT shows focal area of increased tracer uptake consistent with inflammation (SUVmax 5.3). Reproduced from Nanni et al. (2010)

Fig. 7
Ability of Infecton (99mTc-ciprofloxacin) and 99mTc-HMPAO leukocytes in detecting spinal bone lesions. Infecton images reveal hot area with soft tissue extension in L2 and L3 (a). 99mTc-HMPAO-labeled leukocyte study shows a large photopenic area of defect (b). Defects are indicated by arrows. Reproduced from Kumar (2005)
Preliminary findings from our institution in patients with cellulitis and abscess indicated the potential for 68Ga-citrate PET/CT for imaging infection. We have performed a preliminary study in 12 patients using this agent, and the results indicated that infection could be identified within 60 min after injecting 68Ga-citrate, which is a considerable improvement over 67Ga-citrate studies which required 48–72 h post-injection time. However, whether this agent could be used as a first-line agent for imaging infection requires further substantiation. In a patient study, an infected area in the abdomen at the site of recent appendectomy was detected within 30 min post injection of 68Ga-citrate (Fig. 8), which was consistent with CT, and subsequently the abscess (43 mL volume) was drained from the corresponding lesion and microbiology results further confirmed the findings. The SUVmax of the lesion increased from 3.3 to 6.7 from 30 to 60 min (Fig. 8a–c) post-injection period. Cardiac blood pool and liver activities decreased during the period of study. Interestingly, there was persistent high vascular activity in the thigh region. The prolonged vascular retention is consistent with low renal and bladder activity. No bowel activity was seen (Kumar et al. 2012). As the biodistribution of the agent was high in thorax and abdominal region, it has limited but meaningful application (low SUVmax) for imaging infection in these areas but may be ideally suited for imaging infection in the extremities (high SUVmax).
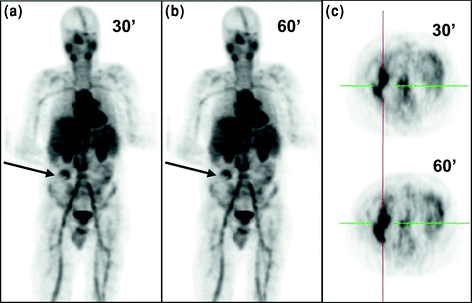

Fig. 8
68Ga-citrate PET imaging of a patient with intra-abdominal infection. 68Ga-citrate (150 MBq) was injected into a patient, and images were acquired at 30 and 60 min intervals. Intra-abdominal infection lesions are shown by arrows. Transverse images are shown in c. High vascular activity and high activity in parotid and salivary glands can be seen in a and b. Reproduced from Kumar et al. (2012)
4.5 68Ga-Apo-Transferrin (68Ga-TF) for PET Imaging of Bacterial Infection
The clinical utility of 67Ga SPECT is somehow compromised due to the delayed post-injection waiting time of at least 24–48 h. In this context, one of the concerns related to 68Ga imaging was the short half-life of 68Ga (t 1/2 = 68 min), which may question the fundamental concept of using this PET radionuclide for longer periods of study. We examined whether the 68Ga-TF complex, once prepared in vitro, is capable of detecting infection faster than 68Ga-citrate (Kumar et al. 2011). This approach might provide circumstantial experimental evidence if the delayed imaging time associated with 67Ga-citrate is due to a longer time required for in vivo binding of Ga3+ to plasma transferrin. TF is a glycoprotein with high affinity for binding iron on its domain. The capability of TF to bind Ga3+ is similar to iron-binding mechanisms (Martinez et al. 1990). Therefore, we prepared 68Ga3+-TF complex in vitro and examined whether this new PET tracer is capable of detecting bacterial infection much faster than 68Ga-citrate. It is important to establish a faster imaging method for 68Ga as its half-life is 68 min compared with 78.3 h for 67Ga.
68Ga-TF was prepared by mixing post-processed pure 68GaCl3 (180 MBq/0.5 mL) with a solution containing 2.0 mg TF in 1.5 mL sodium carbonate (0.1 M, pH 7.0). The reaction vial was incubated in a water bath at 40°C for 1 h. TF used in the experiments was capable of binding up to 1.0 GBq 68GaCl3 under the reaction conditions described. The RCP was >99%. When 68Ga-TF was prepared under identical conditions, except saline was used instead of sodium carbonate solution, the RCP was <15% (Kumar et al. 2011). Therefore, it is mandatory to use sodium bicarbonate instead of saline for preparing 68Ga-TF. The solutions were sterilized for animal injection by final filtering with 0.22-μ filter. The RCP of 68Ga-TF was determined by TLC/alumina chromatography, using sodium citrate (0.1 M) as solvent (free 68Ga, R f = 1; bound 68Ga, R f = 0). Stability study was performed by mixing 0.3 mL 68Ga-TF solution with 1.5 mL human serum at 37°C. RCP was measured at 1 h interval.
When 68Ga-TF (10–15 MBq/0.2 mL) was injected into infected rats, the lesion was detectable within 20 min post injection, although intense, focal uptake was seen only after 50 min post injection (Fig. 9a). The accumulation of activity at the lesion increased with time, as shown by significantly increased SUVmax at the target from 20 min for up to 4 h post injection (Fig. 9b). There was a considerable decrease in cardiac activity during the initial 90 min post-injection period, but a small percentage of activity was measurable for up to 4 h post injection of the study. The activity associated with liver and bowel decreased rapidly up to 90 min of the post-injection period. The low level of activity then persisted for up to 4 h post-injection time. There was marginal increase in the activity associated with the spine over the entire period of the study, whereas muscular uptake increased marginally during the initial 60 min post-injection time but then decreased steadily over the period of the study. Since the background activity was washed out in a time-dependent manner, the T/M ratio increased progressively over the entire period of the study from 2.2 to 7.5 from 10 min to 4 h post injection (Fig. 9b) (Kumar et al. 2011).
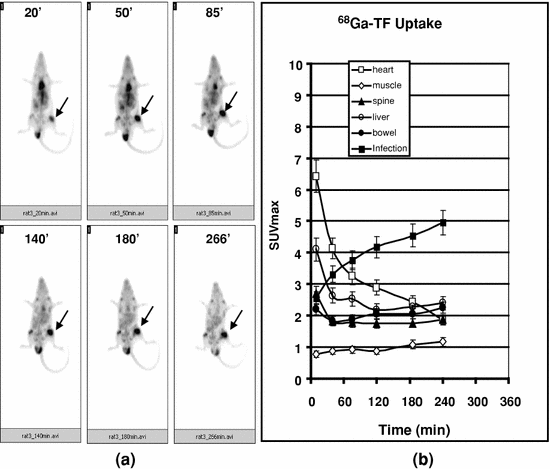

Fig. 9
68Ga-TF uptake by Staph A infection in a rat model. 68Ga-TF (10-15 MBq/0.2 mL) was injected into an anesthetized rat, and images were acquired at different time intervals (up to 4 h). Infection site is indicated by arrows (a). SUVmax was calculated for each time point over different organs and plotted in b. Progressive increase of SUVmax at the target and the concomitant decrease in cardiac blood pool activity could be visualized. Mean ± SD was calculated and plotted. Reproduced from Kumar et al. (2011)
The results in one particular rat showed avid uptake of the agent, as expected at the site corresponding to Staph A-induced infection in the right thigh. It also showed an additional lesion, with clearly defined intense focal uptake in the lower abdominal area, which was persistent during the entire period of study (Fig. 10). Physical examination of the additional lesion site indicated an infected wound and an abscess on the skin. When biopsied, microbiology identified the organism as Proteus mirabilis. Therefore, the study indicated that 68Ga-TF is capable of detecting both Gram-positive and Gram-negative infection caused by both Staph A and Proteus mirabilis, and may be extrapolated to all anaerobic bacteria.
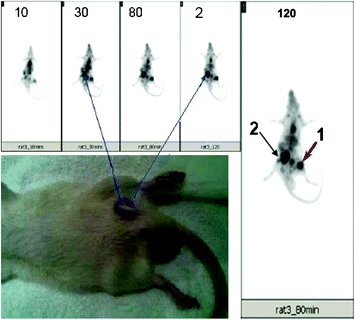

Fig. 10
68Ga-TF uptake by Staph A and an additional infection lesion. 68Ga-TF (10–15 MBq/0.2 mL) was injected into an anesthetized rat, and images were acquired at different time intervals as shown in the figure. Intense uptake was seen at or after 30 min post-injection images at the site corresponding to Staph A injection (arrow 1). An additional intense, focal uptake was seen in the lower right abdominal area, which was identified to be infection due to Proteus mirabilis (arrow 2). Reproduced from Kumar et al. (2011)
Therefore, with the circumstantial experimental evidence, it may be reasonable to conclude that 68Ga-TF complex is able to accumulate at bacterial and inflammatory lesions (Henkin 1978; Tsan 1985). Available literature suggests that the total amount of TF in humans is approximately 240 mg/kg (Bernstein 1998). Therefore, it is reasonable to assume in our study that the mass of injected TF (0.2 mg/rat) in 68Ga-apo-transferrin is too small to induce any unexpected pharmacological effect to influence the biodistribution of the agent.
4.6 Comparison of 68Ga-Chloride and 18F-FDG for PET Imaging of Osteomyelitis and Human Pancreatic Adenocarcinoma Xenografts in Rats
PET using 18F-FDG is ideal for imaging infection, inflammation, and tumor, which are typically characterized by increased glucose metabolism and increased expression of glucose transporters (Ichiya et al. 1996; Sugawara et al. 1999; Stumpe et al. 2000). 18F-FDG uptake is elevated in activated inflammatory cells such as leukocytes, granulocytes, and macrophages because of the above phenomenon. Early bone healing also mimics infection, as it involves an inflammatory phase, which represents a highly activated state of cell metabolism and glucose consumption (Einhorn 1998), and therefore findings with 18F-FDG PET imaging may be complicated by not differentiating inflammation, infection, and bone healing. It has been proposed that an interval of 3–6 months should be allowed to minimize the risk of false-positive findings in patients with postsurgical and traumatic bone healing (de Winter et al. 2002). Recent studies in a rabbit osteomyelitis model indicated that bone infection could be distinguished from bone healing by means of 18F-FDG PET after only an interval of 3 weeks (Koort et al. 2004). However, the transient 18F-FDG uptake by healing bones could still create problems in differential diagnosis under these clinical conditions.
The clinical utility of the two agents 18F-FDG and 68Ga-chloride was compared for monitoring normal bone healing and osteomyelitis in rat tibia (Fig. 11). Quantitative uptake of these agents was also estimated to verify the uptake of 18F-FDG and 68Ga, in normal bone healing and osteomyelitis. The results showed, based on 120-min dynamic imaging, that accumulation of 68Ga was slower (70 min post injection) than that of 18F-FDG (40 min post injection) in the infected bone. On the other hand, they showed increased uptake of both tracers (18F-FDG and 68Ga) in osteomyelitic tibia compared with contralateral normal tibia. Control animals with healing bone defects showed slightly increased uptake of 18F-FDG, but no apparent increase in 68Ga uptake was seen as compared with contralateral intact tibia (Makinen et al. 2005).
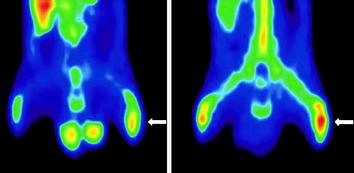

Fig. 11




Comparative 18F-FDG (left) and 68Ga (right) PET coronal images of the lower body in an osteomyelitic animal at 2 weeks. The left infected tibia (white arrow) shows increased tracer uptake for 18F-FDG (SUV ratio 1.8) and 68Ga (SUV ratio 1.6) compared with the intact right tibia. Reproduced from Makinen et al. 2005
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree